S-1: General form for registration of securities under the Securities Act of 1933
Published on June 18, 2025
As filed with the Securities and Exchange Commission on June 17, 2025
Registration No. 333-
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM S-1
REGISTRATION STATEMENT UNDER THE SECURITIES ACT OF 1933
| LIXTE BIOTECHNOLOGY HOLDINGS, INC. |
| (Exact name of registrant as specified in its charter) |
| Delaware | 2834 | 20-2903526 | ||
|
(State or other jurisdiction of incorporation or organization) |
(Primary standard industrial classification code number) |
(I.R.S. employer identification number) |
680 East Colorado Boulevard, Suite 180
Pasadena, CA 91101
(631) 830-7092
(Address, including zip code, and telephone number, including area code, of registrant’s principal executive offices)
Bastiaan van der Baan
Chief Executive Officer
680 East Colorado Boulevard, Suite 180
Pasadena, CA 91101
(631) 830-7092
(Name, address, including zip code, and telephone number, including area code, of agent for service)
Copies to:
|
David L. Ficksman, Esq. TroyGould PC 1801 Century Park East, 16th Floor Los Angeles, CA 90067 Tel: (310) 789-1290 |
Anthony W. Basch, Esq. Kaufman & Canoles Two James Center, 14th Floor Richmond, VA 23219 Tel: (804) 771-5700 |
Approximate date of commencement of proposed sale to the public: As soon as practicable after the effective date of this registration statement.
If any of the securities being registered on this Form are to be offered on a delayed or continuous basis pursuant to Rule 415 under the Securities Act of 1933 check the following box. ☐
If this Form is filed to register additional securities for an offering pursuant to Rule 462(b) under the Securities Act, please check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. ☐
If this Form is a post-effective amendment filed pursuant to Rule 462(c) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. ☐
If this Form is a post-effective amendment filed pursuant to Rule 462(d) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. ☐
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, a smaller reporting company, or an emerging growth company. See the definitions of “large accelerated filer”, “accelerated filer”, “smaller reporting company”, and “emerging growth company” in Rule 12b-2 of the Exchange Act.
| Large accelerated filer | ☐ | Accelerated filer | ☐ |
| Non-accelerated filer | ☒ | Smaller reporting company | ☒ |
| Emerging growth company | ☐ |
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 7(a)(2)(B) of the Securities Act. ☐
The registrant hereby amends this registration statement on such date or dates as may be necessary to delay its effective date until the registrant shall file a further amendment which specifically states that this registration statement shall thereafter become effective in accordance with Section 8(a) of the Securities Act of 1933 or until the registration statement shall become effective on such date as the Securities and Exchange Commission, acting pursuant to said Section 8(a), may determine.
The information in this prospectus is not complete and may be changed. We may not sell these securities until the registration statement filed with the Securities and Exchange Commission is effective. This prospectus is not an offer to sell these securities and it is not soliciting an offer to buy these securities in any state where the offer or sale is not permitted.
The information in this prospectus is not complete and may be changed. The securities may not be sold until the registration statement filed with the SEC is declared effective. This prospectus is not an offer to sell these securities and it is not soliciting an offer to buy these securities in any state where the offer or sale is not permitted.
Subject to Completion, dated June 17, 2025
PRELIMINARY PROSPECTUS
5,263,158 Shares of Common Stock, and/or up to
5,263,158 Pre-Funded Warrants to purchase 5,263,158 Shares of Common Stock
(and 5,263,158 shares of Common Stock underlying the Pre-Funded Warrants)
LIXTE BIOTECHNOLOGY HOLDINGS, INC.
This is a firm commitment public offering of 5,263,158 shares of common stock (“Common Stock”) at an assumed public offering price of $1.14 per share of Common Stock, which is the last reported sales price of our Common Stock on the Nasdaq Capital Market on June 13, 2025.
The public offering price per share of Common Stock will be determined between us and the underwriter based on market conditions at the time of pricing, and may be at a discount to the then current market price of our common stock. Therefore, the recent market price of our common stock referenced throughout this preliminary prospectus may not be indicative of the final offering price per share of Common Stock.
Our common stock is listed on the Nasdaq Capital Market under the symbol “LIXT”. The closing price of our common stock on the Nasdaq Capital Market on June 13, 2025 was $1.14 per share.
We are also offering to investors in shares of Common Stock that would otherwise result in the investor’s beneficial ownership exceeding 4.99% (or, at the election of the purchaser, 9.99%) of our outstanding shares of common stock immediately following the consummation of this offering the opportunity to purchase pre-funded warrants (“Pre-Funded Warrants”) in lieu of Common Stock. Each Pre-Funded Warrant consists of one pre-funded warrant (“Pre-Funded Warrant”) to purchase one share of our common stock. The purchase price of each Pre-Funded Warrant is $1.13999 (which is equal to the assumed public offering price per share of Common Stock of $1.14 minus $0.00001). Subject to limited exceptions, a holder of Pre-Funded Warrants will not have the right to exercise any portion of its Pre-Funded Warrants if the holder, together with its affiliates, would beneficially own in excess of 4.99% (or, at the election of the holder, such limit may be increased to up to 9.99%) of the common stock outstanding immediately after giving effect to such exercise. The Pre-Funded Warrants will be immediately exercisable (subject to the beneficial ownership cap) and may be exercised at any time until all of the Pre-Funded Warrants are exercised in full. For each Pre-Funded Warrant purchased, the number of shares of Common Stock will be decreased on a one-for-one basis. The Pre-Funded Warrants have no stand-alone rights and will not be certificated or issued as stand-alone securities.
We have engaged Spartan Capital Securities, LLC to act as our exclusive underwriter in connection with this offering, hereinafter referred to as the “underwriter” or “Spartan”.
There is no established trading market for the Pre-Funded Warrants and we do not expect an active trading market to develop. We do not intend to list the Pre-Funded Warrants on any securities exchange or other trading market. Without an active trading market, the liquidity of these securities will be limited.
Investing in our securities is speculative and involves a high degree of risk. You should carefully consider the risk factors beginning on page 16 of this prospectus before purchasing our securities.
Neither the Securities and Exchange Commission nor any state securities commission has approved or disapproved of these securities or determined if this prospectus is truthful or complete. Any representation to the contrary is a criminal offense.
| Per Share of Common Stock | Total | |||||||
| Public offering price | $ | $ | ||||||
| Underwriter discount(1) | $ | $ | ||||||
| Proceeds to us, before expenses | $ | $ |
| (1) | We have agreed to pay the underwriter a cash fee equal to 8.0% of the aggregate gross proceeds raised in this offering. We have also agreed to provide the underwriter with a 1.0% non-accountable expense allowance and to reimburse the underwriter for certain of its offering-related expenses, including its legal fees, up to a maximum of $125,000, which are not included in the above table. We have agreed to issue to the underwriter five-year warrants (the “Underwriter Warrants”) to purchase up to 5.0% of the number of shares of Common Stock and the over-allotment option at an exercise price of 125% of the offering price per share of Common Stock. See “Underwriting” for a description of the compensation to be received by the underwriter. |
We have granted the underwriter a 45-day option to purchase up to an additional 789,474 shares of Common Stock, representing 15% of the shares of Common Stock sold in the offering, at an assumed public offering price of $1.14 per share of Common Stock.
The underwriter is expected to deliver the Common Stock (and Pre-Funded Warrants, if any) on or about June __, 2025, subject to the satisfaction of customary closing conditions.
Sole Underwriter
Spartan Capital Securities, LLC
The date of this prospectus is June 17, 2025.
TABLE OF CONTENTS
| i |
You should rely only on the information contained in or incorporated by reference into this prospectus and in any free writing prospectus. We have not authorized anyone to provide you with information different from that contained in this prospectus. The sale of our securities will only be made in jurisdictions where offers and sales are permitted. The information in this prospectus is accurate only as of the date of this prospectus, and any information we have incorporated by reference is accurate only as of the date of the document incorporated by reference, regardless of the time of delivery of this prospectus or any sale of our securities.
We have not done anything that would permit this offering or possession or distribution of this prospectus in any jurisdiction where action for that purpose is required, other than in the United States. Persons outside the United States who come into possession of this prospectus must inform themselves about, and observe any restrictions relating to, the offering of our securities and the distribution of this prospectus outside of the United States.
We own or have rights to trademarks or trade names that we use in connection with the operation of our business, including our corporate names, logos and website names. In addition, we own or have the rights to copyrights, trade secrets and other proprietary rights that protect the content of our products. This prospectus may also contain trademarks, service marks and trade names of other companies, which are the property of their respective owners. Our use or display of third parties’ trademarks, service marks, trade names or products in this prospectus is not intended to, and should not be read to, imply a relationship with or endorsement or sponsorship of us. Solely for convenience, some of the copyrights, trade names and trademarks referred to in this prospectus are listed without their ©, ® and TM symbols, but we will assert, to the fullest extent under applicable law, our rights to our copyrights, trade names and trademarks. All other trademarks are the property of their respective owners.
| 1 |
The following summary highlights information contained or incorporated by reference elsewhere in this prospectus and does not contain all of the information that you should consider in making your investment decision. Before investing in our securities, you should carefully read this entire prospectus, including our consolidated financial statements and the related notes and other documents incorporated by reference herein, as well as the information under the caption “Risk Factors” herein and under similar headings in the other documents that are incorporated by reference into this prospectus including documents that are filed after the date hereof. Some of the statements in this prospectus constitute forward-looking statements that involve risks and uncertainties. See “Cautionary Note Regarding Forward-Looking Statements”. Our actual results could differ materially from those anticipated in such forward-looking statements as a result of certain factors, including those discussed in the “Risk Factors” and other sections included in or incorporated by reference herein. In this prospectus, unless otherwise stated or the context otherwise requires, references to “Lixte”, the “Company”, “we”, “us”, “our”, or similar references mean Lixte Biotechnology Holdings, Inc.
Company Overview
We are a clinical-stage biopharmaceutical company focused on identifying new targets for cancer drug development and developing and commercializing cancer therapies. Our product pipeline is primarily focused on inhibitors of protein phosphatase 2A, which are used to enhance cytotoxic agents, radiation, immune checkpoint blockers and other cancer therapies. We believe that inhibitors of protein phosphatases have significant therapeutic potential for a broad range of cancers. We are focusing on the clinical development of a specific protein phosphatase inhibitor, referred to as LB-100, which has been shown to have clinical anti-cancer activity.
We believe that the mechanism by which LB-100 affects cancer cell growth is different from cancer agents currently approved for clinical use. LB-100 is currently being tested in clinical trials in Ovarian Clear Cell Carcinoma, Metastatic Micro Satellite Stable (MSS) Colon Cancer, and Advanced Soft Tissue Sarcoma. LB-100 has shown anti-cancer activity in animal models of glioblastoma multiforme, neuroblastoma, and medulloblastoma, all cancers of neural tissue. LB-100 has also been shown to enhance the effectiveness of commonly used anti-cancer drugs in animal models of melanoma, breast cancer and sarcoma. The enhancement of anti-cancer activity of these anti-cancer drugs occurs at doses of LB-100 that do not significantly increase toxicity in animals. It is therefore hoped that, when combined with standard anti-cancer regimens against many tumor types, LB-100 will improve therapeutic benefit.
As a compound moves through the FDA-approval process, it becomes an increasingly valuable property, but at a cost of additional investment at each stage. As the potential effectiveness of LB-100 has been documented at the clinical trial level, we have allocated resources to expand the breadth and depth of its patent portfolio. Our approach has been to operate with a minimum of overhead, moving compounds forward as efficiently and inexpensively as possible, and to raise funds to support each of these stages as certain milestones are reached. Our longer-term objective is to secure one or more strategic partnerships or licensing agreements with pharmaceutical companies with major programs in cancer.
Our activities are subject to significant risks and uncertainties, including the need for additional capital. We have not yet commenced any revenue-generating operations, does not have positive cash flows from operations, relies on stock-based compensation for a substantial portion of employee and consultant compensation, and is dependent on periodic access to equity capital to fund its operating requirements.
Description of Business
Most cancer patients are treated with either chemotherapy or immunotherapy or both. These therapies often have limited benefit and there is a high unmet medical need to enhance their effects. In many preclinical models we have shown that LB-100 enhances the effect of both chemotherapy and Immunotherapy
| 2 |

LB-100, a small molecule potent inhibitor of PP2A, was designed and developed by us. Numerous preclinical studies have documented that LB-100 potentiates most if not all anti-cancer drugs that damage DNA. LB-100 is not associated with any increase in cytotoxicity when given with cytotoxic drugs. This synergy involves transient interruption of several DNA damage repair pathways by LB-100 and an increase in cell division rate. LB-100 has FDA Investigational New Drug status in the US and Investigational Medicinal Product Dossier approval in the European Union.
In its initial Phase 1 clinical trial, LB-100 given alone daily for 3 days was non-toxic, except for a transient increase in serum creatinine believed to be caused by inhibition of PP2A in the renal tubules. In the Phase 1 clinical trial, the Maximum Tolerated Dose (“MTD”) was 2.33mg/m2 daily for 3 days every 3 weeks. Of the 25 patients with heavily-treated advanced solid tumors with measurable disease, 3 patients had stable disease for 2 cycles, 3 patients had stable disease for 4 cycles, and 3 patients had stable disease for 6 cycles. One patient with pancreatic cancer had a partial response after 12 cycles lasting 534 days.
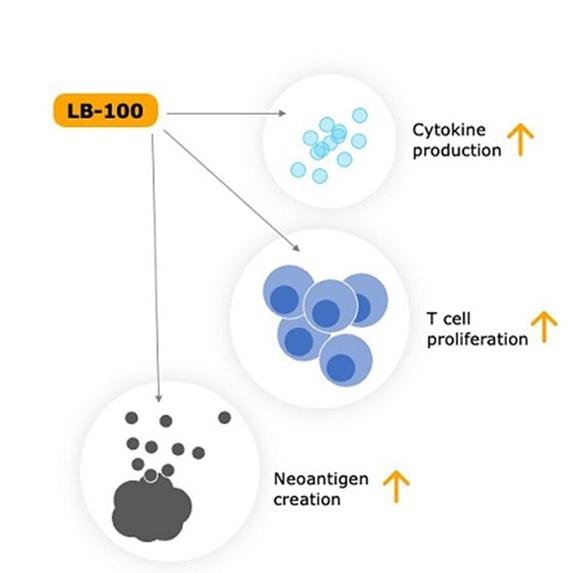
Low doses of LB-100 have now been shown to enhance immune checkpoint inhibition (“ICI”) by several different mechanisms affecting the tumor compartment and immune T-cell compartment. LB-100 increases CD8+T-cell infiltration and CD8-Treg ratio, CD8+T-cell proliferation, and cytokine production induces microsatellite instability, neoantigen production and immune responsiveness, converting immunologically “cold” to “hot” cancers.
| 3 |
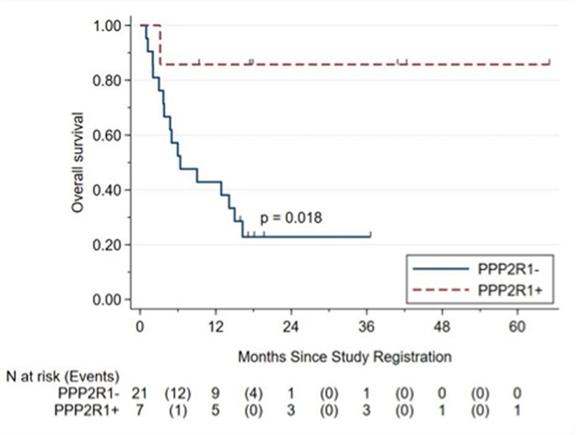
Ovarian clear cell carcinoma patients with inactivating mutations in PPP2R1A, a gene coding for a scaffold component of PP2A, and treated with immune checkpoint inhibitors, were recently found to have markedly longer survival than patients without the mutation in their cancers. Retrospective reviews of patients with a variety of cancers treated with ICI or chemotherapy show much longer survival of ICI-treated patients with a PPP2R1A mutation in their tumors.
| 4 |
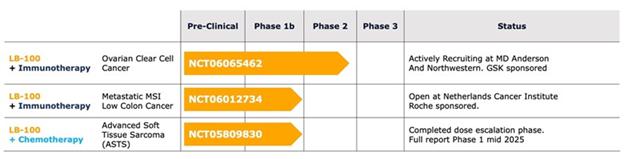
Based on the observations in ovarian clear cell carcinoma, we have initiated a clinical trial in this disease combining LB-100 with a monoclonal antibody blocking PD-1, a protein found on T-cells (NCT06065462).
Given these preclinical and clinical observations, it is likely that LB-100 may be a general way to enhance immunotherapy responses.
The research on the LB-100 series was initiated in 2006 under a Cooperative Research and Development Agreement (“CRADA”) with the National Institute of Neurologic Disorders and Stroke or NINDS of the National Institutes of Health or NIH dated March 22, 2006 that was subsequently extended through a series of amendments until it terminated on April 1, 2013.
We have also designed and developed the LB-200 series, which consists of histone deacetylase inhibitors (HDACi). LB-200 has not advanced to the clinical stage and would require additional capital to fund further development. Accordingly, because of our focus on the clinical development of LB-100 and analogs for cancer therapy as described below in more detail, we have decided not to actively pursue the preclinical development of our LB-200 series of compounds at this time.
| 5 |
Clinical Trial Agreements
Spanish Sarcoma Group Collaboration Agreement
Effective July 31, 2019, we entered into a Collaboration Agreement for an Investigator-Initiated Clinical Trial with the Spanish Sarcoma Group (Grupo Español de Investigación en Sarcomas or “GEIS”), Madrid, Spain, to carry out a study entitled “Randomized phase I/II trial of LB-100 plus doxorubicin vs. doxorubicin alone in first line of advanced soft tissue sarcoma”. The purpose of this clinical trial is to obtain information with respect to the efficacy and safety of LB-100 combined with doxorubicin in soft tissue sarcomas. Doxorubicin is the global standard for initial treatment of advanced soft tissue sarcomas (“ASTS”). Doxorubicin alone has been the mainstay of first line treatment of ASTS for over 40 years, with little improvement in survival from adding cytotoxic compounds to or substituting other cytotoxic compounds for doxorubicin. In animal models, LB-100 consistently enhances the anti-tumor activity of doxorubicin without apparent increases in toxicity.
GEIS has a network of referral centers in Spain and across Europe that have an impressive track record of efficiently conducting innovative studies in ASTS. We agreed to provide GEIS with a supply of LB-100 to be utilized in the conduct of this clinical trial, as well as to provide funding for the clinical trial. The goal is to enter approximately 150 to 170 patients in this clinical trial over a period of two to four years. The Phase 1 portion of the study began in the quarter ended June 30, 2023 to determine the recommended Phase 2 dose of the combination of doxorubicin and LB-100. As advanced sarcoma is a very aggressive disease, the design of the Phase 2 portion of the study assumes a median progression-free survival (“PFS”), no evidence of disease progression or death from any cause) of 4.5 months in the doxorubicin arm and an alternative median PFS of 7.5 months in the doxorubicin plus LB-100 arm to demonstrate a statistically significant decrease in relative risk of progression or death by adding LB-100. There is a planned interim analysis of the primary endpoint when approximately 50% of the 102 events required for final analysis is reached.
On October 13, 2022, we announced that the Spanish Agency for Medicines and Health Products (Agencia Española de Medicamentos y Productos Sanitarios or “AEMPS”) had authorized a Phase 1b/randomized Phase 2 study of LB-100, our lead clinical compound, plus doxorubicin, versus doxorubicin alone, the global standard for initial treatment of advanced soft tissue sarcomas (ASTS). Consequently, this clinical trial commenced during the quarter ended June 30, 2023 and to be completed and a report prepared by December 31, 2026. In April 2023, GEIS completed its first site initiation visit in preparation for the clinical trial at Fundación Jiménez Díaz University Hospital (Madrid). Up to 170 patents will be entered into the clinical trial. The recruitment phase of the Phase 1b portion of the protocol was completed during the quarter ended September 30, 2024. We expect to have data on toxicity and preliminary efficacy from this portion of the clinical trial during the quarter ending December 31, 2025.
Given the focus on the combination of LB-100 with immunotherapy in ovarian clear cell carcinoma and colorectal cancer and the availability of capital resources, we entered into Amendment No. 1 to the Collaboration Agreement effective March 11, 2025 that relieved us of the financial obligation to support the randomized Phase 2 portion of the clinical trial contemplated in the Collaboration Agreement of approximately $3,095,000. As a result, it is uncertain as to whether the Phase 2 portion of this clinical trial will proceed.
Clinical Research Support Agreement Relating to Small Cell Lung Cancer
We had executed a Clinical Research Support Agreement with the City of Hope National Medical Center to carry out a Phase 1b clinical trial of LB-100 combined with an FDA-approved standard regiment for treatment of untreated extensive-stage disease small cell lung cancer. The clinical trial was initiated on March 9, 2021. However, due to the lack of patient accrual, the Company provided notice to the City of Hope National Medical Center of our intent to terminate the Clinical Research Support Agreement effective as of July 8, 2024.
| 6 |
MD Anderson Cancer Center Clinical Trial
On September 20, 2023, we announced an investigator-initiated Phase 1b/2 collaborative clinical trial to assess whether adding LB-100 to a human programmed death receptor-1 (“PD-1”) blocking antibody of GSK plc (“GSK”), dostarlimab-gxly, may enhance the effectiveness of immunotherapy in the treatment of ovarian clear cell carcinoma (“OCCC”). The clinical trial is being sponsored by The University of Texas MD Anderson Cancer Center (“MD Anderson”) and is being conducted at The University of Texas – MD Anderson Cancer Center. We are providing LB-100 and GSK is providing dostarlimab-gxly and financial support for the clinical trial. On January 29, 2024, we announced the entry of the first patient into this clinical trial. We currently expect that this clinical trial will be completed by December 31, 2027.
On February 25, 2025, we announced that we had added the Robert H. Lurie Comprehensive Cancer Center (Lurie Cancer Center) of Northwestern University as a second site in a clinical trial combining the Company’s proprietary compound LB-100 with GSK’s dostarlimab to treat ovarian clear cell cancer. Patient recruitment is underway, and the first patient has been dosed.
Netherlands Cancer Institute Clinical Trial
Effective June 10, 2024, we entered into a Clinical Trial Agreement with the Netherlands Cancer Institute (“NKI”) to conduct a Phase 1b clinical trial of the Company’s protein phosphatase inhibitor, LB-100, combined with atezolizumab, a PD-L1 inhibitor, the proprietary molecule of F. Hoffman-La Roche Ltd. (“Roche”), for patients with microsatellite stable metastatic colon cancer. Under the agreement, we will provide our lead clinical compound, LB-100, and under a separate agreement between NKI and Roche, Roche will provide atezolizumab and financial support for the clinical trial. We have no obligation to and will not provide any reimbursement of clinical trial costs. Pursuant to the agreement and the protocol set forth in the agreement, the clinical trial will be conducted by NKI at NKI’s site in Amsterdam by principal investigator Neeltje Steeghs, MD, PhD, and NKI will be responsible for the recruitment of patients. The agreement provides for the protection of the respective intellectual property rights of each of Lixte, NKI and Roche.
This Phase 1b clinical trial will evaluate safety, optimal dose and preliminary efficacy of LB-100 combined with atezolizumab for the treatment of patients with metastatic microsatellite stable colorectal cancer. Immunotherapy using monoclonal antibodies like atezolizumab can enhance the body’s immune response against cancer and hinder tumor growth and spread. LB-100 has been found to improve the effectiveness of anticancer drugs in killing cancer cells by inhibiting a protein called PP2A on cell surfaces. Blocking PP2A increases stress signals in tumor cells expressing the PP2A protein. Accordingly, combining atezolizumab with LB-100 may enhance treatment efficacy for metastatic colorectal cancer, as cancer cells with heightened stress signals are more vulnerable to immunotherapy.
This study comprises a dose escalation phase and a dose expansion phase. The objective of the dose escalation phase is to determine the recommended Phase 2 dose (RP2D) of LB-100 when combined with the standard dosage of atezolizumab. The dose expansion phase will further investigate the preliminary efficacy, safety, tolerability, and pharmacokinetics/dynamics of the LB-100 and atezolizumab combination. The clinical trial opened in August 2024 with the enrollment of the first patient. Patient accrual is expected to take up to 24 months, with a maximum of 37 patients with advanced colorectal cancer to be enrolled in this study.
The shelf life of the batch of LB-100 being utilized in this clinical trial was scheduled to expire on December 25, 2025, but has been extended for a final time for a period of 12 months through December 25, 2026, after which date no new patients can be recruited into this clinical trial and no patients can be treated with the current batch of LB-100. Although we do not currently intend to commission the production of a new batch of LB-100 for this clinical trial, we believe that it is likely that we will be able to recruit enough patients in sufficient time into this clinical trial to be able to reach an evaluable outcome for all end points in this clinical trial by December 25, 2026. The expiration of the shelf life of this batch of LB-100 represents an effective termination date of this clinical trial.
The principal investigator of the colorectal study testing LB-100 in combination with atezolizumab is currently investigating two Serious Adverse Events (“SAEs”) observed in the clinical trial that was launched in August 2024. The Investigational Review Board (IRB) of the Netherlands Cancer Institute has requested additional information with respect to these SAEs and the study has been paused for enrollment until the IRB’s questions have been, as more fully discussed below at “Risks Related to the Development and Regulatory Approval of Our Product Candidates - A clinical trial hold due to serious adverse events could delay or halt the development of our product candidate”.
| 7 |
National Cancer Institute Pharmacologic Clinical Trial
In May 2019, the National Cancer Institute (NCI) initiated a glioblastoma (GBM) pharmacologic clinical trial. This study was being conducted and funded by the NCI under a Cooperative Research and Development Agreement, with the Company being required to provide the LB-100 clinical compound.
Primary malignant brain tumors (gliomas) are very challenging to treat. Radiation combined with the chemotherapeutic drug temozolomide has been the mainstay of therapy of the most aggressive gliomas (glioblastoma multiforme or GBM) for decades, with little further benefit gained by the addition of one or more anti-cancer drugs, but without major advances in overall survival for the majority of patients. In animal models of GBM, the Company’s novel protein phosphatase inhibitor, LB-100, has been found to enhance the effectiveness of radiation, temozolomide chemotherapy treatments and immunotherapy, raising the possibility that LB-100 may improve outcomes of standard GBM treatment in the clinic. Although LB-100 has proven safe in patients at doses associated with apparent anti-tumor activity against several human cancers arising outside the brain, the ability of LB-100 to penetrate tumor tissue arising in the brain was not known. Many drugs potentially useful for GBM treatment do not enter the brain in amounts necessary for anti-cancer action.
The NCI study was designed to determine the extent to which LB-100 enters recurrent malignant gliomas. Patients having surgery to remove one or more tumors received one dose of LB-100 prior to surgery and had blood and tumor tissue analyzed to determine the amount of LB-100 present and to determine whether the cells in the tumors showed the biochemical changes expected to be present if LB-100 reached its molecular target. As a result of the innovative design of the NCI study, it was believed that data from a few patients would be sufficient to provide a sound rationale for conducting a larger clinical trial to determine the effectiveness of adding LB-100 to the standard treatment regimen for GBMs. Blood and brain tumor tissue were analyzed from seven patients after intravenous infusion of a single dose of LB-100. Results of the investigation demonstrated that there was virtually no entry of LB-100 into the brain tumor tissue. Accordingly, alternative methods of drug delivery will be required to determine if LB-100 has meaningful clinical anti-cancer activity against glioblastoma multiforme and other aggressive brain tumors.
Patent and License Agreements
National Institute of Health
Effective February 23, 2024, we entered into a Patent License Agreement (the “License Agreement”) with the National Institute of Neurological Disorders and Stroke (“NINDS”) and the National Cancer Institute (“NCI”), each an institute or center of the National Institute of Health (“NIH”). Pursuant to the License Agreement, we have licensed exclusively NIH’s intellectual property rights claimed for a Cooperative Research and Development Agreement (“CRADA”) subject invention co-developed with the Company, and the licensed field of use, which focuses on promoting anti-cancer activity alone, or in combination with standard anti-cancer drugs. The scope of this clinical research extends to checkpoint inhibitors, immunotherapy, and radiation for the treatment of cancer. The License Agreement is effective, and shall extend, on a licensed product, licensed process, and country basis, until the expiration of the last-to-expire valid claim of the jointly owned licensed patent rights in each such country in the licensed territory, unless sooner terminated.
The License Agreement contemplates that we will seek to work with pharmaceutical companies and clinical trial sites (including comprehensive cancer centers) to initiate clinical trials within timeframes that will meet certain benchmarks. Data from the clinical trials will be the subject of various regulatory filings for marketing approval in applicable countries in the licensed territories. Subject to the receipt of marketing approval, we would be expected to commercialize the licensed products in markets where regulatory approval has been obtained.
Other Significant Agreements and Contracts
Netherlands Cancer Institute
| 8 |
On October 8, 2021, we entered into a Development Collaboration Agreement with the Netherlands Cancer Institute, Amsterdam (“NKI”), one of the world’s leading comprehensive cancer centers, and Oncode Institute, Utrecht, a major independent cancer research center, for a term of three years. The Development Collaboration Agreement was subsequently modified by Amendment No. 1 thereto.
The Development Collaboration Agreement is a preclinical study intended to identify the most promising drugs to be combined with LB-100, and potentially LB-100 analogues, to be used to treat a range of cancers, as well as to identify the specific molecular mechanisms underlying the identified combinations. We agreed to fund the preclinical study, at an approximate cost of 391,000 Euros and provide a sufficient supply of LB-100 to conduct the preclinical study.
On October 3, 2023, we entered into Amendment No. 2 to the Development Collaboration Agreement with NKI, which provides for additional research activities, extends the termination date of the Development Collaboration Agreement by two years to October 8, 2026, and added 500,000 Euros to the operating budget being funded by us.
On October 4, 2024, we entered into Amendment No. 3 to the Development Collaboration Agreement with NKI, which suspended Amendment No. 2 and provided for a new study term of one year and starts upon the dosing of the first patient in the clinical trial at a project cost of 100,000 Euros.
Effective as of June 15, 2022, Dr. René Bernards was appointed to our Board of Directors as an independent director. Dr. Bernards is a leader in the field of molecular carcinogenesis and is employed by NKI.
Intellectual Property
Our intellectual property includes proprietary know-how, proprietary methodologies and extensive clinical validation data and publications. To provide legal protection of our intellectual property, we rely on a combination of patents, licenses, trade secrets, trademarks, confidentiality and non-disclosure clauses and agreements, and other forms of intellectual property protection to define and protect our rights to our products.
Our products are expected to be covered by our patents. These patents now cover sole rights to the composition and synthesis of our LB-100 series of drugs, which is the Company’s lead clinical compound in development. Lixte has filed patent applications covering the treatment of cancer with LB-100. Lixte has also filed joint patent applications with the NIH and the Netherlands Cancer Institute for the treatment of cancer using LB-100 in combination with other drugs like immune checkpoint inhibitors and WEE1 inhibitors (a class of drugs that target and inhibit the WEE1 kinase enzyme that plays a crucial role in regulating cell division).
Patent applications for the LB-100 series (oxabicycloheptanes and oxabicycloheptenes) have been filed in the United States and internationally under the Patent Cooperation Treaty. Patents for composition of matter and for several uses of the LB-100 series have been issued in the United States, Mexico, Australia, Japan, China, Hong Kong, Canada, and by the European Patent Office
We strive to protect and enhance the proprietary technology, inventions, and improvements that are commercially important to the development of our business, including seeking, maintaining, and defending its patent rights, which are owned solely by our wholly-owned Delaware subsidiary, Lixte Biotechnology, Inc., except in several instances jointly with one of many of our collaborators. We also rely on trade secrets relating to its proprietary pipeline of product candidates and on know-how and continuing technological innovation to develop and strengthen its pipeline. We intend to rely on regulatory protection afforded by regulatory agencies through data exclusivity, market exclusivity, and patent term extensions, where available.
Our success will depend in large part on its ability to obtain and maintain patent and other proprietary protection for commercially important technology, inventions and know-how related to its business; defend and enforce its patents; preserve the confidentiality of its trade secrets; and operate without infringing valid and enforceable patents or proprietary rights of third parties. Our ability to stop third parties from making, using, selling, offering to sell, or importing our technology may depend on the extent to which we have rights under valid and enforceable licenses, patents, or trade secrets that cover these activities. In some cases, enforcement of these rights may depend on cooperation of the joint owners of our jointly owned patents and patent applications.
| 9 |
With respect to both our solely and jointly owned intellectual property, we cannot be sure that patents will be granted on any of its pending patent applications or on any patent applications filed solely or jointly by us in the future; we cannot be sure that any of our existing patents or any patents that may be granted to us in the future will be commercially useful in protecting our intended commercial products or therapeutic methods; and we cannot be sure that an agency or court would determine that the our solely or jointly owned patents are valid and enforceable.
Nasdaq Compliance
On August 19, 2024, we received a letter from the Listing Qualifications Department (the “Staff”) of the Nasdaq Stock Market LLC indicating that we were not in compliance with the minimum stockholders’ equity requirement of $2,500,000 for continued listing on the Nasdaq Capital Market under Listing Rule 5550(b) (the “Equity Rule”).
On October 3, 2024, we submitted a plan to the Staff to regain compliance with the Equity Rule, which outlined our proposed initiatives to regain compliance by raising equity capital through various registered equity offerings.
On October 21, 2024, the Staff provided notice to us that it had granted an extension through February 18, 2025 to regain compliance with the Equity Rule.
As of February 18, 2025, we had not regained compliance with the Equity Rule. Accordingly, on February 19, 2025, we received a Staff determination letter from the Staff stating that we did not meet the terms of the extension because we did not complete our proposed financing initiatives to regain compliance. We timely requested a Hearing before a Nasdaq Hearings Panel (the “Panel”), which automatically stayed Nasdaq’s suspension or delisting of our Common Stock and public warrants pending the Panel’s decision.
On April 17, 2025, we received notice that the Panel had granted us an extension in which to regain compliance with all continued listing rules of the Nasdaq Capital Market. The Panel’s determination followed a hearing on April 3, 2025, at which time the Panel considered our plan to regain compliance with the Equity Rule. As a result of the extension, the Panel granted our request for continued listing on the Nasdaq Capital Market, provided that we demonstrate compliance with the Equity Rule and all other continued listing requirements for the Nasdaq Capital Market by July 3, 2025.
This offering is being conducted in order for us to satisfy the decision of the Panel and to provide the working capital resources to fund our operations. However, there can be no assurances that the Panel will deem the proceeds from this offering as sufficient to comply with the Equity Rule and all other continued listing requirements for the Nasdaq Capital Market. Accordingly, even if we complete this offering, there can be no assurances that we will be able to regain compliance during the extension period and thus be able to maintain our listing on the Nasdaq Capital Market.
During the extension period, our Common Stock and public warrants will continue to trade on the Nasdaq Capital Market.
Corporate Information
We were incorporated as a Delaware Corporation on May 24, 2005 under the name SRKP7, Inc. On June 30, 2006, pursuant to a share exchange agreement, we acquired all of the outstanding shares of Lixte Biotechnology, Inc. which then became a wholly owned subsidiary. On December 7, 2006, we changed our name to Lixte Biotechnology Holdings, Inc.
Effective September 26, 2023, Bastiaan van der Baan, a director of the Company since June 17, 2022, replaced our founder, John S. Kovach, as President and Chief Executive Officer. Dr. Kovach passed away on October 5, 2023. Effective October 6, 2023, Mr. van der Baan was appointed as Chairman of the Board of Directors.
| 10 |
As discussed below, effective June 16, 2025, Mr. van der Baan resigned as Chairman of the Board of Directors and Chief Executive Officer, but remained as President and as a member of the Board of Directors, and was appointed as the Company’s Chief Scientific Officer, and Geordan Pursglove was appointed as Chairman of the Board of Directors and Chief Executive Officer.
Our common stock and public warrants are traded on the Nasdaq Capital Market under the symbols “LIXT” and “LIXTW”, respectively. On June 2, 2023, we effected a one-for-ten reverse split of our outstanding shares of common stock in order to remain in compliance with the $1.00 minimum closing bid price requirement of the Nasdaq Capital Market.
Our principal address is 680 East Colorado Boulevard, Suite 180, Pasadena, CA 91101. Our telephone number is (631) 830-7092. We maintain a website at https://lixte.com. The information contained on our website is not, and should not be interpreted to be, incorporated into this prospectus.
July 2023 Financing
On July 20, 2023, we sold 583,334 shares of common stock at a price of $6.00 per share to an institutional investor and raised gross proceeds of approximately $3,500,000. As part of this financing, we sold warrants to the institutional investor to purchase 583,334 shares of common stock (the “2023 Warrants”). The 2023 Warrants had an initial exercise price of $6.00 per share, were immediately exercisable upon issuance, and expire five years thereafter on July 20, 2028. We also issued warrants to the placement agent to purchase 35,000 shares of common stock at an exercise price of $6.60 per share and expiring on July 20, 2028 (the “2023 Placement Agent Warrants”).
The exercise prices of the warrants issued to the institutional investor and to the placement agent are subject to customary adjustments for stock splits, stock dividends, stock combinations, reclassifications, reorganizations, or similar events affecting our common stock. In addition, the warrants issued to the institutional investor contain a “fundamental transaction” provision whereby in the event of a fundamental transaction (including a sale or transfer of assets or ownership of the Company as defined in the warrant agreement) within our control, the holder of the unexercised common stock warrants would be entitled to receive, in exchange for extinguishment of the warrants, cash consideration equal to a Black-Scholes valuation, as defined in the warrant agreement. If such fundamental transaction is not within our control, the warrant holder would only be entitled to receive the same form of consideration (and in the same proportion) as the holders of our common stock.
Accordingly, in the event of a change in control of the Company or a sale or transfer of all or substantially all of our assets, as defined in the 2023 Warrants, to the extent that the warrants issued to the institutional investor are outstanding at the effective date that such a transaction is closed, this “fundamental transaction” provision would entitle the holder to substantial cash consideration, thus reducing the amounts to be retained by us or potentially distributable to our stockholders.
February 2025 Financing
On February 11, 2025, we entered into a Securities Purchase Agreement (the “Purchase Agreement”) with two institutional investors (the “Selling Stockholders”). Pursuant to the Purchase Agreement, on February 13, 2025, we sold 434,784 shares of common stock at a price of $2.415 per share and raised gross proceeds of approximately $1,050,000. As part of this financing, we sold warrants to the institutional investors to purchase 434,784 shares of common stock (the “2025 Warrants”). Such Warrants had an initial exercise price of $2.29 per share, were immediately exercisable upon issuance, and expire five years thereafter on February 12, 2030. We also issued warrants to the placement agent to purchase 32,609 shares of common stock at an exercise price of $3.0188 per share and expiring on February 12, 2030 (the “2025 Placement Agent Warrants”).
The exercise prices of the warrants issued to the institutional investors and to the placement agent are subject to customary adjustments for stock splits, stock dividends, stock combinations, reclassifications, reorganizations, or similar events affecting our common stock. In addition, the warrants issued to the institutional investors contain a “fundamental transaction” provision whereby in the event of a fundamental transaction (including a sale or transfer of assets or ownership of the Company as defined in the warrant agreement) within our control, the holders of the unexercised common stock warrants would be entitled to receive, in exchange for extinguishment of the warrants, cash consideration equal to a Black-Scholes valuation, as defined in the warrant agreement. If such fundamental transaction is not within our control, the warrant holders would only be entitled to receive the same form of consideration (and in the same proportion) as the holders of our common stock.
| 11 |
Accordingly, in the event of a change in control of the Company or a sale or transfer of all or substantially all of our assets, as defined in the 2025 Warrants, to the extent that the warrants issued to the institutional investors are outstanding at the effective date that such a transaction is closed, this “fundamental transaction” provision would entitle the holders to substantial cash consideration, thus reducing the amounts to be retained by us or potentially distributable to our stockholders.
Executive Management and Director Changes
Prior to and effective as of the consummation of this public offering, there will be several changes with respect to our executive management and the Board of Directors as described herein.
Bastiaan van der Baan. Effective June 16, 2025, Bastiaan van der Baan resigned as Chairman of the Board of Directors and as Chief Executive Officer, but remained as President and as a member of the Board of Directors, and was appointed as Chief Scientific Officer. Mr. van der Baan’s principal responsibility as President will be related to the clinical development of our LB-100 lead compound. His responsibility as Chief Scientific Officer (CSO) will be for shaping and executing the scientific vision and research and development strategy of the Company, with a focus on discovering and developing innovative cancer therapeutics. The CSO leads all research functions, oversees preclinical and translational programs, supervises the Chief Medical Officer, and ensures alignment with clinical and regulatory development goals. The CSO provides scientific leadership to internal teams and external partners, supports fundraising and business development, and serves as a key member of executive management.
In conjunction with such resignation, the stock option that Mr. van der Baan was previously granted on September 26, 2023 to acquire 250,000 shares of common stock was deemed fully vested effective with Mr. van der Baan’s resignation as described herein, and the time period for Mr. van der Baan to exercise his stock option at any time in the future that he is no longer providing his services to the Company as a consultant, employee or otherwise was increased from ninety (90) days to one (1) year.
Except for the previously described changes in Mr. van der Baan’s management duties and the modifications to the terms of the stock option, Mr. van der Baan’s three (3) year employment agreement dated September 26, 2023 will remain in full force and effect.
If the Company does not complete a successful financing that enables it to maintain its listing on the Nasdaq Small Cap Market by July 3, 2025, the amendment to Mr. van der Baan’s employment agreement as described herein will be automatically terminated retroactive to the amendment date and Mr. van der Baan will be reinstated as Chairman of the Board of Directors and Chief Executive Officer.
Geordan Pursglove. Effective June 16, 2025, Geordan Pursglove was appointed as our new Chairman of the Board of Directors and Chief Executive Officer. His responsibilities include the oversight of our business operations and strategic planning, and he will be the primary contact between our executive team and the Board of Directors. He will also be the principal spokesperson of the Company and have final say on all corporate matters, subject only to the authority of the Board of Directors.
We have entered into an employment agreement with Mr. Pursglove for a term of three (3) years effective June 16, 2025. Mr. Pursglove will receive an annual salary of $240,000 which may be increased from time to time in the sole discretion of the Board of Directors. At his election, his compensation will be payable in cash and/or restricted shares, or a combination thereof.. He is also eligible to receive an annual bonus as determined in the sole discretion of the Board of Directors in the form of cash or equity, or a combination thereof. Mr. Pursglove will not receive any additional compensation for serving as the Chairman of the Board of Directors of the Company.
Effective as of the end of the first trading day for the Company’s common stock immediately following the consummation of this offering, as an inducement to Mr. Pursglove to join our Company, as a signing bonus, he will be granted a stock option to purchase 350,000 shares of our common stock at an exercise price equal to the closing price on the Nasdaq Stock Market on such date, which shall be exercisable for a term of five (5) years, shall provide for cashless exercise, and shall vest 50% on the grant date, 25% on September 30, 2025, and 25% on December 31, 2025, subject to continued service.
| 12 |
The stock option grant will not be issued under the Company’s 2020 Stock Incentive Plan. The stock option agreement will provide for certain registration rights (including on Form S-8) and for accelerated vesting upon the occurrence of certain events, including early termination of the employment agreement that is not the result of the voluntary termination, gross negligence or willful misconduct of Mr. Pursglove, a sale or change in control of the Company, or a sale, licensing or other disposition of all or substantially all of the assets of the Company, as defined in such stock option agreement.
If the Company does not complete a successful financing that enables it to maintain its listing on the Nasdaq Capital Market by July 3, 2025, the employment agreement with Mr. Pursglove as described herein will be deemed automatically terminated retroactively as of June 16, 2025 and the stock option grant will be cancelled, and Mr. Pursglove will promptly resign from the Board of Directors. In such event, Mr. van der Baan will be reinstated as Chairman of the Board of Directors and Chief Executive Officer.
Prior to joining the Company, Mr. Pursglove served as President, Chief Executive Officer and Chairman of the Board of Directors at Beyond Commerce, Inc. He was also President of Service 800, Inc., a leading phone and online customer satisfaction survey service that provides the most actionable customer feedback, to the most recognizable Fortune 500 companies globally, in which he led operations, scaled revenue, and oversaw the company’s strategic vision. Mr. Pursglove held a board position at SemiCab Holdings, an emerging leader in the global logistics and distribution industry that was part of Algorhythm Holdings (NASDAQ: RIME). Currently Mr. Pursglove serves as the managing director of The 2GP Group LLC. During his time as the Managing Director of The 2GP Group, Mr. Pursglove has built multiple businesses in Sports, Sales, Marketing and Logistics. Mr. Pursglove has over a decade of experience in M&A, public markets space, capital raising, funding, growth, scaling businesses and driving innovation.
Peter Stazzone. Effective upon the closing of the public offering, Peter Stazzone will be appointed as a member of the Board of Directors and as the Chair of the Audit Committee, replacing Regina Brown, the current Chair of the Audit Committee, who will resign from the Board of Directors effective upon Mr. Stazzone’s appointment. Mr. Stazzone is a senior finance and business development executive with over 20 years of experience in finance and operations management within start-ups, high-growth and multi-billion dollar organizations. Mr. Stazzone is an experienced board member in both the public and non-profit sectors. He earned his Master of Business Administration (Finance) from DePaul University and his Bachelor of Science (Accounting) from the University of Illinois. He also is a member of the American Institute of Certified Public Accountants. From 2021 to the present, Mr. Stazzone has acted as the Chief Financial Officer of Beyond Commerce, Inc., a publicly traded company operating in the Business-to-Business Internet Marketing Technology and Services, electric vehicles and logistics markets. From 2016 to 2021, Mr. Stazzone was the Chief Financial Officer of Strainz, Inc., a leading cannabis brand and manufacturing company operating in Colorado, Washington, and Nevada.
| 13 |
| Issuer: | Lixte Biotechnology Holdings, Inc. | |
| Securities offered by us: |
5,263,158 shares of Common Stock
We are also offering to investors in shares of Common Stock that would otherwise result in the investor’s beneficial ownership exceeding 4.99% (or, at the election of the purchaser, 9.99%) of our outstanding shares of common stock immediately following the consummation of this offering the opportunity to purchase Pre-Funded Warrants in lieu of Common Stock. Each Pre-Funded Warrant consists of one pre-funded warrant (“Pre-Funded Warrant”) to purchase one share of our common stock. The purchase price of each Pre-Funded Warrant is $1.13999 (which is equal to the assumed public offering price per share of Common Stock of $1.14 minus $0.00001). Subject to limited exceptions, a holder of Pre-Funded Warrants will not have the right to exercise any portion of its Pre-Funded Warrants if the holder, together with its affiliates, would beneficially own in excess of 4.99% (or, at the election of the holder, such limit may be increased to up to 9.99%) of the common stock outstanding immediately after giving effect to such exercise. The Pre-Funded Warrants will be immediately exercisable (subject to the beneficial ownership cap) and may be exercised at any time until all of the Pre-Funded Warrants are exercised in full. For each Pre-Funded Warrant purchased, the number of Common Stock we are offering will be decreased on a one-for-one basis. The Pre-Funded Warrants have no stand-alone rights and will not be certificated or issued as stand-alone securities.
|
|
| Over-Allotment Option: | The offering is being underwritten on a firm commitment basis. We have granted the underwriter a 45-day option to purchase up to an additional 789,474 shares of Common Stock, representing 15% of the shares of Common Stock sold in the offering, at an assumed public offering price of $1.14 per share of Common Stock. | |
| Underwriter Warrants: |
We have also granted the underwriter five-year warrants to purchase up to 5.0% of the shares of Common Stock and Pre-Funded Warrants including in the over-allotment option at an exercise price of 125% of the offering price per share of Common Stock. The Underwriter Warrants may not be exercised, sold, transferred, assigned, pledged or hypothecated, or be the subject of any hedging, short sale, derivative, put, or call transaction that would result in the effective economic disposition of such securities for a period of one hundred eighty (180) days beginning on the commencement of sales of the offering.
|
|
| Assumed public offering price: | $1.14 per share of Common Stock, which is the closing price of our common stock on the Nasdaq Capital Market on June 13, 2025. | |
| Common stock outstanding immediately prior to this offering: | 2,756,991 shares of common stock. | |
| Common stock to be outstanding immediately after this offering: | 8,020,149 shares(1) of our common stock (or 8,809,623 shares of common stock if the over-allotment option is exercised in full) assuming no issuance of Pre-Funded Warrants, and no exercise of any of the Underwriter Warrants issued in this offering). | |
| Use of proceeds: | We currently intend to use the net proceeds from this offering for working capital and general corporate purposes. See “Use of Proceeds”. |
| 14 |
| Underwriting: | Spartan proposes to offer the shares of Common Stock purchased pursuant to the underwriting agreement between us and Spartan to the public at the public offering price set forth on the cover page of this prospectus. In addition, we will reimburse Spartan for certain out-of-pocket expenses, including legal fees, related to the offering up to a maximum of $125,000. See “Underwriting”. | |
| Nasdaq Capital Market trading symbol: | Our common stock currently trades on the Nasdaq Capital Market under the symbol “LIXT”. We do not intend to list the Pre-Funded Warrants offered hereunder on any stock exchange. | |
| Transfer agent and registrar: | The transfer agent and registrar for our common stock is Computershare Trust Company, N.A. | |
| Risk factors: | The securities offered by this prospectus are speculative and involve a high degree of risk. Investors purchasing securities should not purchase the securities unless they can afford the loss of their entire investment. See “Risk Factors”. |
| (1) | Immediately after this offering, the Company will have 8,020,149 shares of common stock outstanding as set forth above, assuming that the underwriter over-allotment option is not exercised, and will exclude: |
| ● | 662,078 shares of common stock issuable upon the exercise of common stock options issued to members of management, consultants and directors at a weighted average exercise price of $11.526 per share; | |
| ● | 1,275,758 shares of common stock issuable upon exercise of outstanding common stock warrants at an average exercise price of $11.254 per share of common stock, including 434,784 shares of common stock issuable upon exercise of 434,784 common stock warrants exercisable at $2.29 per share issued in our February 13, 2025 offering, 32,609 shares of common stock issuable upon exercise of 32,609 common stock warrants exercisable at $3.1088 per share issued to the placement agent in our February 13, 2025 offering, and 149,700 shares of common stock issuable upon exercise of 149,700 publicly traded warrants at $57.00 per share of common stock through November 30, 2025; | |
| ● | 133,339 shares of common stock reserved for future grants pursuant to our 2020 Stock Incentive Plan, as amended (the “2020 Plan”); and | |
| ● | 263,158 shares of common stock issuable upon exercise of the Underwriter Warrants at an exercise price of $1.425 per share of common stock issuable to the underwriter in this offering, assuming that the over-allotment option is not exercised. |
Unless otherwise indicated, this prospectus also assumes no sale of Pre-Funded Warrants, no exercise of any of the Underwriter Warrants, and no exercise of the underwriter over-allotment option.
| 15 |
Investing in our common stock is highly speculative and involves a significant degree of risk. You should carefully consider the following risks and uncertainties as well as the risks and uncertainties described in the section entitled “Risk Factors” contained in our Annual Report on Form 10-K for the fiscal year ended December 31, 2024 and in our Quarterly Report on Form 10-Q for the quarterly period ended March 31, 2025, filed with the Securities and Exchange Commission, which filings are incorporated in this prospectus by reference in their entirety, as well as in any prospectus supplement hereto. These risk factors could materially and adversely affect our business, results of operations or financial condition. Our business faces significant risks and the risks described below or incorporated by reference herein may not be the only risks we face. Additional risks not presently known to us or that we currently believe are immaterial may materially affect our business, results of operations, or financial condition. If any of these risks occur, the trading price of our common stock could decline and you may lose all or part of your investment.
Risks Related to the Development and Regulatory Approval of Our Product Candidates
A clinical trial hold due to serious adverse events could delay or halt the development of our product candidate.
Our lead drug candidate, LB-100, is currently undergoing various clinical trials, and there is a risk that one or more of these trials could be placed on hold by regulatory authorities due to serious adverse events (SAEs) related to our drug candidate or to another company’s drug used in combination in one of our clinical trials. It is possible that the SAEs could be attributable to our drug candidate and could include, but not be limited to, unexpected severe side effects, treatment-related deaths, or long-term health complications. A dose given could result in non-tolerable adverse events defined as dose-limiting toxicity (DLT). When two DLTs occur at the same dose-level that dose-level is considered too high and unsafe. Further treatment is only allowed at lower dose-levels that have previously been found safe.
If an SAE or a pattern of SAEs is observed during the course of a clinical trial involving our drug candidate, the U.S. Food and Drug Administration (FDA), European Medicines Agency (EMA), or other regulatory authorities may issue a clinical hold, requiring us to pause or discontinue further enrollment and dosing in our clinical trial. It is also possible that the clinical trial could be terminated. Any of these actions could delay or halt the development of our drug candidate, increase development costs, and negatively impact our ability to ultimately achieve regulatory approval. Additionally, if an SAE is confirmed to be drug-related, we may be required to conduct additional studies, modify the study design, or abandon further development of the drug candidate altogether, which could materially impact our business, financial condition, and prospects.
The occurrence of an SAE and any resulting clinical hold could also harm our reputation with patients, physicians, health institutions, and investors, diminish our ability to attract clinical trial participants, and damage our ability to interest investors and obtain financing in the future. There can be no assurances that we will not experience such SAEs in the future or that any related clinical hold will be lifted in a timely manner, or at all.
The principal investigator of the colorectal study testing LB-100 in combination with atezolizumab (Roche PD-L1 inhibitor) is currently investigating two SAEs observed in the clinical trial that was launched in August 2024. The Institutional Review Board (the “IRB”) of the Netherlands Cancer Institute (“NKI”) has put the colorectal cancer study on hold. The adverse reactions that developed in the two patients were dyspnea (shortness of breath) due to lung toxicity possibly or probably related to the combination of LB-100 and atezolizumab in one patient and fever and aphasia possibly or probably related to the combination of LB-100 and atezolizumab in the second patient. The patient who developed lung toxicity deceased due to the combination of lung metastases of colorectal cancer and dyspnea. The patient with fever and aphasia fully recovered from the adverse events with supportive medication.
Given the identified adverse events in the two patients in the clinical trial, the IRB requested from the principal investigator of the study at the NKI information as to whether the adverse events could have been caused by the combination of LB-100 and atezolizumab and information about the mode of action of the combination of LB-100 and atezolizumab. The principal investigator prepared a response to the IRB detailing the safety experience with LB-100 given alone and in combination with other cancer drugs, especially doxorubicin and dostarlimab. Doxorubicin is a well-known chemotherapy, and dostarlimab is a well-known immunotherapy of which the mode of action is closely related to that of atezolizumab.
| 16 |
The reported adverse events in the colorectal cancer study have not been seen in any other patients thus far treated with LB-100 alone or in combination with other cancer drugs. Through May 2025, the Company has been informed that a total of 79 patients had received or were receiving experimental treatment with LB-100.
In May 2025, the Company updated the safety overview of LB-100 and delivered the updated version 5.0 of the Investigator’s Brochure (the “IB”), which contains all of the relevant preclinical, clinical and pharmacologic data with respect to the study of the LB-100 clinical compound in humans, to the investigators of all ongoing clinical trials. The investigators of the study in colorectal cancer (NCT06012734) submitted a detailed response to the IRB, including the updated IB. The Company is currently awaiting the outcome of the IRB review.
Risks Related to this Offering and Ownership of our Securities
We have a history of losses, expect to continue to incur losses in the near term and may not achieve or sustain profitability in the future, and as a result, our management has identified, and our auditors agreed that there is a substantial doubt about our ability to continue as a going concern.
We have incurred significant losses since our inception. We experienced net losses of $3,585,965 and $5,087,029 for the years ended December 31, 2024 and 2023, respectively, and $709,555 and $971,322 for the three months ended March 31, 2025 and 2024, respectively. We expect our operating losses will continue, or even increase, at least through the near term. You should not rely upon our past results as indicative of future performance. We will not reach profitability in the near future or at any specific time in the future.
The report of our independent registered public accounting firm that accompanies our audited consolidated financial statements in our Annual Report on Form 10-K for the fiscal year ended December 31, 2024 contains an explanatory paragraph regarding substantial doubt about our ability to continue as a going concern. Our consolidated financial statements do not include any adjustments that might result if we are unable to continue as a going concern. If we are unable to continue as a going concern, holders of our securities might lose their entire investment.
We are currently not in compliance with the Nasdaq Capital Market’s continued listing requirements. If we are unable to regain compliance with the Nasdaq Capital Market’s listing requirements, our securities could be delisted, which could affect our common stock’s market price and liquidity and reduce our ability to raise capital.
On August 19, 2024, we received a letter from the Listing Qualifications Department (the “Staff”) of the Nasdaq Stock Market LLC indicating that we were not in compliance with the minimum stockholders’ equity requirement of $2,500,000 for continued listing on the Nasdaq Capital Market under Listing Rule 5550(b) (the “Equity Rule”).
On October 3, 2024, we submitted a plan to the Staff to regain compliance with the Equity Rule, which outlined our proposed initiatives to regain compliance by raising equity capital through various registered equity offerings.
On October 21, 2024, the Staff provided notice to us that it had granted an extension through February 18, 2025 to regain compliance with the Equity Rule.
As of February 18, 2025, we had not regained compliance with the Equity Rule. Accordingly, on February 19, 2025, we received a Staff determination letter from the Staff stating that we did not meet the terms of the extension because we did not complete our proposed financing initiatives to regain compliance. We timely requested a Hearing before a Nasdaq Hearings Panel (the “Panel”), which automatically stayed Nasdaq’s suspension or delisting of our Common Stock and public warrants pending the Panel’s decision.
On April 17, 2025, we received notice that the Panel had granted us an extension in which to regain compliance with all continued listing rules of the Nasdaq Capital Market. The Panel’s determination followed a hearing on April 3, 2025, at which time the Panel considered our plan to regain compliance with the Equity Rule. As a result of the extension, the Panel granted our request for continued listing on the Nasdaq Capital Market, provided that we demonstrate compliance with the Equity Rule and all other continued listing requirements for the Nasdaq Capital Market by July 3, 2025.
| 17 |
This offering is being conducted in order for us to satisfy the decision of the Panel and to provide the working capital resources to fund our operations. However, there can be no assurances that the Panel will deem the proceeds from this offering as sufficient to comply with the Equity Rule and all other continued listing requirements for the Nasdaq Capital Market. Accordingly, even if we complete this offering, there can be no assurances that we will be able to regain compliance during the extension period and thus be able to maintain our listing on the Nasdaq Capital Market.
During the extension period, our Common Stock and public warrants will continue to trade on the Nasdaq Capital Market.
We cannot assure you that we will be able to regain compliance with the Nasdaq Capital Market’s listing standards. Our failure to continue to meet these requirements would result in our common stock being delisted from Nasdaq, and if our common stock is delisted, the warrants issued in our public offering would also be delisted. We and holders of our securities could be materially adversely impacted if our securities are delisted from the Nasdaq Capital Market. In particular:
| ● | we may be unable to raise equity capital on acceptable terms or at all; | |
| ● | we may lose the confidence of our clinical partners, which would jeopardize our ability to continue our clinical trials as currently conducted; | |
| ● | the price of our common stock will likely decrease as a result of the loss of market efficiencies associated with the Nasdaq Capital Market and the loss of federal pre-emption of state securities laws; | |
| ● | holders may be unable to sell or purchase our securities when they wish to do so; | |
| ● | we may become subject to stockholder litigation; | |
| ● | we may be unable to attract, or we may lose the interest of, institutional investors in our common stock; | |
| ● | we may lose media and analyst coverage; | |
| ● | our common stock could be considered a “penny stock”, which would likely limit the level of trading activity in the secondary market for our common stock; and | |
| ● | we would likely lose any active trading market for our common stock, as it may only be traded on one of the over-the-counter markets, if at all. |
We will have to seek to raise additional funds to fund our operations, including the various clinical trials being currently conducted or will be conducted in the future. Depending on the terms available to us, if these fund raising activities result in significant dilution, they may negatively impact the trading price of our common stock.
Any additional financing that we secure may require the granting of rights, preferences or privileges senior to, or pari passu with, those of our common stock. Any issuances by us of equity securities may be at or below the prevailing market price of our common stock and in any event may have a dilutive impact on your ownership interest, which could cause the market price of our common stock to decline. We may also raise additional funds through the incurrence of debt or the issuance or sale of other securities or instruments senior to our shares of common stock, which may be highly dilutive. The holders of any securities or instruments we may issue may have rights superior to the rights of our common stockholders. If we experience dilution from the issuance of additional securities and we grant superior rights to new securities over holders of our common stock, it may negatively impact the trading price of our common stock and you may lose all or part of your investment.
As part of the Company’s ongoing process of evaluating various alternatives to obtain the capital required to fund its operations and maintain its listing on the Nasdaq Capital Market, management may decide to consider a wide variety of strategic alternatives, and there can be no assurances that any such transaction, if implemented, would enhance stockholder value, and could be highly dilutive to existing stockholders.
| 18 |
The Company is evaluating various alternatives to obtain the capital required to fund its operations and maintain its listing on the Nasdaq Capital Market, including merger or acquisition opportunities (including reverse mergers) and funding transactions involving a change in control. There can be no assurances that the evaluation process will result in the identification of an appropriate transaction, the negotiation and execution of a definitive agreement to effect such a transaction, or that any such transaction will ultimately be approved by the Company’s stockholders and then be consummated. Depending on various factors, many of which are outside the control of the Company, our failure to enter into and consummate a strategic transaction could have a material adverse effect on our ability to continue to operate and finance our business, and on the market price of our common stock. Even if such a strategic transaction is consummated, there can be no assurances that it will enhance stockholder value, and it may result in substantial dilution to existing stockholders. Any potential transaction would be dependent on a number of factors that may be outside of our control, including, among other things, market conditions, industry trends, the interest of third parties in a potential transaction with the Company, and the availability of appropriate financing for such a transaction. If we are unable to raise the required capital to fund our operations, or to enter into a strategic transaction in the near future, we may not be able to maintain our listing on the Nasdaq Capital Market, and we may need to curtail or cease operations, which could result in a total loss of stockholders’ investment.
The price of our common stock might fluctuate substantially.
You should consider an investment in our common stock to be risky. Some factors that might cause the market price of our common stock or public warrants to fluctuate, in addition to the other risks mentioned in this “Risk Factors” section, are:
| ● | sale of our common stock by our stockholders, executives, and directors and our stockholders; | |
| ● | volatility and limitations in trading volumes of our shares of common stock; | |
| ● | our ability to obtain financings to conduct and complete research and development activities including, but not limited to, our clinical trials, and other business activities; | |
| ● | the timing and success of introductions of new products by us or our competitors or any other change in the competitive dynamics of our industry, including consolidation among competitors, customers or strategic partners; | |
| ● | network outages or security breaches; | |
| ● | our ability to secure resources and the necessary personnel to conduct clinical trials on our desired schedule; | |
| ● | commencement, enrollment or results of our clinical trials for our lead product candidate or any future clinical trials we might conduct; | |
| ● | changes in the development status of our lead product candidate; | |
| ● | any delays or adverse developments or perceived adverse developments with respect to the FDA’s review of our planned preclinical and clinical trials; | |
| ● | any delay in our submission for studies or product approvals or adverse regulatory decisions, including failure to receive regulatory approval for our lead product candidate; | |
| ● | unanticipated safety concerns related to the use of our lead product candidate; | |
| ● | failures to meet external expectations or management guidance; | |
| ● | changes in our capital structure or dividend policy, future issuances of securities, sales of large blocks of common stock by our stockholders; | |
| ● | our cash position; |
| 19 |
| ● | announcements and events surrounding financing efforts, including debt and equity securities; | |
| ● | our inability to enter into new markets or develop new products; | |
| ● | reputational issues; | |
| ● | competition from existing technologies and products or new technologies and products that might emerge; | |
| ● | announcements of acquisitions, partnerships, collaborations, joint ventures, new products, capital commitments, or other events by us or our competitors; | |
| ● | changes in general economic, political and market conditions in or any of the regions in which we conduct our business; | |
| ● | changes in industry conditions or perceptions; | |
| ● | changes in valuations of similar companies or groups of companies; | |
| ● | analyst research reports, recommendations and changes in recommendations, price targets, and withdrawals of coverage; | |
| ● | departures and additions of key personnel; | |
| ● | disputes and litigations related to intellectual properties, proprietary rights, and contractual obligations; | |
| ● | changes in applicable laws, rules, regulations, or accounting practices and other dynamics; and | |
| ● | other events or factors, many of which might be out of our control. |
In addition, if the market for stocks in our industry or industries related to our industry, or the stock market in general, experiences a loss of investor confidence, the trading price of our common stock could decline for reasons unrelated to our business, financial condition and results of operations. If any of the foregoing occurs, it could cause our stock price to fall and might expose us to lawsuits that, even if unsuccessful, could be costly to defend and a distraction to management.
Provisions of the Warrants issued in the 2023 Financing and 2025 Financing could discourage an acquisition of us by a third party.
Certain provisions of the 2023 Warrants and 2025 Warrants could make it more difficult or expensive for a third party to acquire us. Such Warrants prohibit us from engaging in certain transactions constituting “fundamental transactions” unless, among other things, the surviving entity assumes our obligations under such Warrants. These could prevent or deter a third party from acquiring us even where the acquisition could be beneficial to you. Also, we may be required to redeem these Warrants for a cash payment calculated pursuant to the Black-Scholes option-pricing model.
An active, liquid and orderly trading market for our common stock may not develop, the price of our stock may be volatile, and you could lose all or part of your investment.
Even though our common stock is currently listed on the Nasdaq Capital Market, we cannot predict the extent to which investor interest in our Company will lead to the development of an active trading market in our securities or how liquid that market might become. If such a market does not develop or is not sustained, it may be difficult for you to sell your shares of common stock at the time that you wish to sell them, at a price that is attractive to you, or at all. There could be extreme fluctuations in the price of our common stock if there are a limited number of shares in our public float.
| 20 |
The trading price of our common stock may be highly volatile and could be subject to wide fluctuations in response to various factors, some of which are beyond our control. Our stock price could be subject to wide fluctuations in response to a variety of factors, which include:
| ● | whether we achieve our anticipated corporate objectives; | |
| ● | actual or anticipated fluctuations in our quarterly or annual operating results; | |
| ● | changes in our financial or operational estimates; | |
| ● | our ability to implement our operational plans; | |
| ● | changes in the economic performance or market valuations of companies similar to ours; and | |
| ● | general economic or political conditions in the United States or elsewhere. |
In addition, broad market and industry factors may seriously affect the market price of companies’ stock, including ours, regardless of actual operating performance. In the past, following periods of volatility in the overall market and the market price of a particular company’s securities, securities class action litigation has often been instituted against these companies. This litigation, if instituted against us, could result in substantial costs and a diversion of our management’s attention and resources.
Our outstanding Warrants may cause the trading price of our common stock to decrease.
Depending on the price of our common stock, the number of shares of common stock issuable pursuant to the exercise of the Warrants issued in the 2023 Financing and the 2025 Financing could result in an immediate decrease in the trading price of our common stock. If the bid price of our common stock falls below $1.00 per share for thirty (30) consecutive business days, we would no longer meet the Nasdaq Capital Market’s minimum bid price requirement and our common stock would be subject to delisting. We cannot predict the effect, if any, that the availability of shares for future sale represented by such Warrants will have on the trading price of our common stock from time to time.
If our shares of common stock become subject to the penny stock rules, it would become more difficult to trade our shares.
The Securities and Exchange Commission has adopted rules that regulate broker-dealer practices in connection with transactions in penny stocks. Penny stocks are generally equity securities with a price of less than $5.00, other than securities registered on certain national securities exchanges or authorized for quotation on certain automated quotation systems, provided that current price and volume information with respect to transactions in such securities is provided by the exchange or system. If we do not retain a listing on the Nasdaq Capital Market and if the price of our common stock is less than $5.00, our common stock will be deemed a penny stock. The penny stock rules require a broker-dealer, before a transaction in a penny stock not otherwise exempt from those rules, to deliver a standardized risk disclosure document containing specified information. In addition, the penny stock rules require that before effecting any transaction in a penny stock not otherwise exempt from those rules, a broker-dealer must make a special written determination that the penny stock is a suitable investment for the purchaser and receive (i) the purchaser’s written acknowledgment of the receipt of a risk disclosure statement; (ii) a written agreement to transactions involving penny stocks; and (iii) a signed and dated copy of a written suitability statement. These disclosure requirements may have the effect of reducing the trading activity in the secondary market for our common stock, and therefore stockholders may have difficulty selling their shares.
If we were to dissolve, the holders of our securities may lose all or substantial amounts of their investments.
If we were to dissolve as a corporation, as part of ceasing to do business or otherwise, we will be required to pay all amounts owed to any creditors before distributing any assets to holders of our capital stock. There is a risk that in the event of such a dissolution, there will be insufficient funds to repay amounts owed to holders of any of our indebtedness and insufficient assets to distribute to our capital stockholders, in which case investors could lose their entire investment.
If securities or industry analysts do not publish or cease publishing research or reports about us, our business or our market, or if they change their recommendations regarding our securities adversely, our stock price and trading volume could decline.
| 21 |
The trading market for our common stock is influenced by the research and reports that industry or securities analysts may publish about us, our business, our market or our competitors. If any of the analysts who may cover us change their recommendation regarding our common stock adversely, or provide more favorable relative recommendations about our competitors, our stock price would likely decline. If any of the analysts who may cover us were to cease coverage of our Company or fail to regularly publish reports on us, we could lose visibility in the financial markets, which in turn could cause our stock price or trading volume to decline.
In making your investment decision, you should understand that we have not authorized any other party to provide you with information concerning us or this offering.
You should carefully evaluate all of the information in this prospectus before investing in our company. We may receive media coverage regarding our company, including coverage that is not directly attributable to statements made by our officers, that incorrectly reports on statements made by our officers or employees, or that is misleading as a result of omitting information provided by us, our officers or employees. We and the underwriter have not authorized any other party to provide you with information concerning us or this offering, and you should not rely on unauthorized information in making an investment decision.
The common stock and Pre-Funded Warrants (which are exercisable for common stock) sold in this offering will increase the number of our shares of common stock from 2,756,991 shares to 8,020,149 shares. If the Underwriter’s over-allotment option is exercised, the number of our shares of common stock will increase by an additional 789,474 shares. The sales of these securities could depress the market price of our shares of common stock and/or increase the volatility of our trading.
A substantial number of shares of common stock and Pre-Funded Warrants are being offered by this prospectus. Sales of a substantial number of our shares of common stock in the public markets pursuant to the terms of this offering could depress the market price of our shares of common stock and impair our ability to raise capital to the sale of additional equity securities. Additionally, such sales could also greatly increase the volatility associated with the trading of our common stock. We cannot predict the number of shares that might be sold, nor the effect future sales of shares would have on the market price of our shares.
The Pre-Funded Warrants are speculative in nature and there is not expected to be a trading market for the Pre-Funded Warrants.
There is no established trading market for the Pre-Funded Warrants and we do not expect a trading market to develop. Without a trading market, the liquidity of the Pre-Funded Warrants will be limited.
Holders of the Pre-Funded Warrants will have no rights as a common stockholder until they acquire our common stock.
The Pre-Funded Warrants offered in this offering do not confer any rights of common stock ownership on their holders, such as voting rights or the right to receive dividends, but rather merely represent the right to acquire shares of our common stock at a fixed price for a limited period of time. In the case of Pre-Funded Warrants, holders may exercise their right to acquire the common stock and pay an exercise price of $0.00001 per share. The Pre-Funded Warrants do not expire. Until holders of the Pre-Funded Warrants acquire shares of our common stock upon exercise of the Pre-Funded Warrants, the holders will have no rights with respect to shares of our common stock issuable upon exercise of the Pre-Funded Warrants. Upon exercise of the Pre-Funded Warrants, the holder will be entitled to exercise the rights of a common stockholder as to the security exercised only as to matters for which the record date occurs after the exercise.
Investors in this offering will experience immediate and substantial dilution in the book value of their investment.
The public offering price will be substantially higher than the net tangible book value per share of our outstanding shares of common stock. As a result, investors in this offering will incur immediate dilution of $[ ] per share based on the assumed public offering price of $[ ] per share of Common Stock. Investors in this offering will pay a price per share of Common Stock that substantially exceeds the book value of our assets after subtracting our liabilities. See “Dilution” for a more complete description of how the value of your investment will be diluted upon the completion of this offering.
| 22 |
A possible “short squeeze” due to a sudden increase in demand of our shares of common stock that largely exceeds supply may lead to price volatility in our shares of common stock.
Following this offering, investors may purchase our shares of common stock to hedge existing exposure in our shares of common stock or to speculate on the price of our shares of common stock. Speculation on the price of our shares of common stock may involve long and short exposures. To the extent aggregate short exposure exceeds the number of shares of our common stock available for purchase in the open market, investors with short exposure may have to pay a premium to repurchase our shares of common stock for delivery to lenders of our shares of common stock. Those repurchases may in turn, dramatically increase the price of our shares of common stock until investors with short exposure are able to purchase additional shares of Common Stock to cover their short position. This is often referred to as a “short squeeze”. A short squeeze could lead to volatile price movements in our shares of common stock that are not directly correlated to the performance or prospects of our company and once investors purchase the shares of common stock necessary to cover their short position the price of our common stock may decline.
CAUTIONARY NOTE REGARDING FORWARD-LOOKING STATEMENTS
This prospectus, and the documents incorporated by reference herein may contain forward-looking statements within the meaning of Section 27A of the Securities Act of 1933, as amended (the “Securities Act”), and Section 21E of the Securities Exchange Act of 1934, as amended (the “Exchange Act”). All statements other than statements of historical facts contained in this prospectus, including statements regarding our future results of operations and financial position, business strategy and plans and our objectives for future operations, are forward-looking statements. The words “anticipate”, “believe”, “could”, “estimate”, “expect”, “forecast”, “intend”, “may”, “plan”, “potential”, “should”, “will”, “would”, “might”, and similar expressions are intended to identify forward-looking statements. These forward-looking statements are subject to certain risks and uncertainties that could cause actual results to differ materially from historical results or anticipated results, including:
| ● | We are engaged in early-stage research and as such might not be successful in our efforts to develop a portfolio of commercially viable products; | |
| ● | We have incurred substantial losses since our inception and anticipate that we will continue to incur substantial and increasing losses for the foreseeable future; | |
| ● | Our independent registered public accounting firm has expressed substantial doubt about our ability to continue as a going concern; | |
| ● | We need significant additional financing to fund our operations and complete the development and, if approved, the commercialization of our lead product candidate, LB-100. If we are unable to raise capital when needed, we could be forced to delay, reduce or eliminate our product development programs or commercialization efforts; | |
| ● | We currently have no source of revenues. We might never generate revenues or achieve profitability; | |
| ● | Our ability to use net operating losses to offset future taxable income might be subject to limitations; | |
| ● | Clinical-stage biopharmaceutical companies with product candidates in clinical development face a wide range of challenging activities which might entail substantial risk; | |
| ● | We might find it difficult to enroll patients in our clinical trials which could delay or prevent the start of clinical trials for our product candidate; | |
| ● | The results of preclinical studies or earlier clinical trials are not necessarily predictive of future results. Our lead product candidate in clinical trials, and any other product candidates that might advance into clinical trials, might not have favorable results in later clinical trials or receive regulatory approval; | |
| ● | Clinical drug development involves a lengthy and expensive process with an uncertain outcome; | |
| ● | There is a risk that one or more of our clinical trials could be placed on hold by regulatory authorities due to serious adverse events (SAEs) related to our drug candidate or to another company’s drug used in combination in one of our clinical trials. It is possible that the SAEs could be attributable to our drug candidate and could include, but not be limited to, unexpected severe side effects, treatment-related deaths, or long-term health complications. A dose given could result in non-tolerable adverse events defined as dose-limiting toxicity (DLT). When two DLTs occur at the same dose-level that dose-level is considered too high and unsafe. Further treatment is only allowed at lower dose-levels that have previously been found safe. |
| 23 |
| ● | Risks associated with operating in foreign countries could materially adversely affect our product development; | |
| ● | Our current and future product candidates, the methods used to deliver them or their dosage levels may cause undesirable side effects or have other properties that could delay or prevent their regulatory approval, limit the commercial profile of an approved label or result in significant negative consequences following any regulatory approval; | |
| ● | Our product development program might not uncover all possible adverse events that patients who take our lead product candidate may experience. The number of subjects exposed to our lead product candidate and the average exposure time in the clinical development program might be inadequate to detect rare adverse events or chance findings that might only be detected once the product is administered to more patients and for greater periods of time; | |
| ● | Our future success is dependent on the regulatory approval of our lead product candidate; | |
| ● | Our lead product candidate and future product candidates could fail to receive regulatory approval from the FDA; | |
| ● | Failure to obtain regulatory approval in international jurisdictions would prevent our lead product candidate from being marketed abroad; | |
| ● | Even if our current primary product candidate receives regulatory approval, it might still face future development and regulatory difficulties; | |
| ● | We depend on certain key scientific personnel for our success who do not work full time for us. The loss of any such personnel could adversely affect our business, financial condition and results of operations; | |
| ● | We expect to rely heavily on third parties for the conduct of clinical trials of our product candidates. If these clinical trials are not successful, or if we or our collaborators are not able to obtain the necessary regulatory approvals, we will not be able to commercialize our product candidates; | |
| ● | Business interruptions could adversely affect future operations, revenues, and financial conditions, and might increase our costs and expenses; | |
| ● | Our failure to find third party collaborators to assist or share in the costs of product development could materially harm our business, financial condition or results of operations; | |
| ● | We might be subject to claims by third parties asserting that our employees, consultants, collaborators contractors or we have misappropriated their intellectual property, or claiming ownership of what we regard as our own intellectual property; | |
| ● | We cannot be certain we will be able to obtain patent protection to protect our product candidates and technology; | |
| ● | If we do not obtain patent term extension in the United States under the Hatch-Waxman Act or in foreign countries under similar legislation, our business might be materially harmed; | |
| ● | If we fail to comply with our obligations in agreements under which we have licensed or, might license, intellectual property rights from third parties, or if we otherwise experience disruptions to our business relationships with our licensors, we could lose rights that are important to our business; | |
| ● | We might infringe the intellectual property rights of others, which may prevent or delay our product development efforts and stop us from commercializing or increase the costs of commercializing our product candidates; | |
| ● | We might be subject to claims that our employees, consultants or independent contractors have wrongfully used or disclosed alleged trade secrets of one or more third parties; | |
| ● | Our intellectual property might not be sufficient to protect our intended products from competition, which might negatively affect our business as well as limit our partnership or acquisition appeal; | |
| ● | If we are not able to protect and control our unpatented trade secrets, know-how and other technological innovation, we might suffer competitive harm; |
| 24 |
| ● | We might incur substantial costs prosecuting our patent applications, maintaining our patents and patent applications, enforcing our patents, defending against third party patent infringement suits, seeking invalidation of third party patents or in-licensing third party intellectual property, as a result of litigation or other proceedings relating to patent and other intellectual property rights; | |
| ● | If we are unable to protect our intellectual property rights, our competitors might develop and market products with similar or identical features that might reduce demand for our potential products; | |
| ● | Our commercial success depends upon attaining significant market acceptance of our current product candidate and future product candidates, if approved, among physicians, patients, healthcare payors and cancer treatment centers; | |
| ● | Even if we are able to commercialize our lead product candidate or any future product candidates, the products might not receive coverage or adequate reimbursement from third party payors in the United States and in other countries in which we seek to commercialize our intended products, which could harm our business; | |
| ● | Healthcare legislative measures aimed at reducing healthcare costs might have a material adverse effect on our business and results of operations; | |
| ● | Price controls might be imposed in foreign markets, which might adversely affect our future profitability; | |
| ● | Our relationships with customers and third party payors will be subject to applicable anti-kickback, fraud and abuse and other healthcare laws and regulations, which could expose us to criminal sanctions, civil penalties, contractual damages, reputational harm and diminished profits and future earnings. If we or they are unable to comply with these provisions, we might become subject to civil and criminal investigations and proceedings that could have a material adverse effect on our business, financial condition and prospects; | |
| ● | Our employees might engage in misconduct or other improper activities, including noncompliance with regulatory standards and requirements, which could cause significant liability for us and harm our reputation; | |
| ● | Product liability lawsuits against us could cause us to incur substantial liabilities and to limit commercialization of any products that we might develop; | |
| ● | We face substantial competition, which might result in others discovering, developing or commercializing products before or more successfully than we do; | |
| ● | Significant disruptions of information technology systems, computer system failures or breaches of information and cyber security could adversely affect our business; | |
| ● | We might need to grow the size of our organization in the future, and we might experience difficulties in managing this growth; | |
| ● | Inadequate funding for the FDA, the SEC and other government agencies could hinder their ability to hire and retain key leadership and other personnel, prevent new products and services from being developed or commercialized in a timely manner or otherwise prevent those agencies from performing normal business functions on which the operation of our business might rely, which could negatively impact our business; | |
| ● | Unstable market and economic conditions and adverse developments with respect to financial institutions and associated liquidity risk may have serious adverse consequences on our business, financial condition and stock price; | |
| ● | We are a “smaller reporting company” and we have elected to comply with certain reduced reporting and disclosure requirements which could make its common stock less attractive to investors; | |
| ● | The price of our common stock might fluctuate substantially; | |
| ● | A sale or perceived sale of a substantial number of shares of our common stock might cause the price of our common stock to decline; | |
| ● | Market and economic conditions might negatively impact our business, financial condition and share price; | |
| ● | If securities or industry analysts do not publish research or reports, or publish unfavorable research or reports about our business, our stock price and trading volume might decline; | |
| ● | Future sales and issuances of our common stock could result in additional dilution of the percentage ownership of our stockholders and could cause our share price to fall; | |
| ● | We do not intend to pay cash dividends on our shares of Common Stock so any returns will be limited to the value of our shares; |
| 25 |
| ● | We might be at risk of securities class action litigation; | |
| ● | Our Certificate of Incorporation and our Amended and Restated Bylaws, and Delaware law might have anti-takeover effects that could discourage, delay or prevent a change in control, which might cause our stock price to decline; | |
| ● | Financial reporting obligations of being a public company in the United States are expensive and time-consuming, and our management will be required to devote substantial time to compliance matters; and | |
| ● | If we fail to comply with the rules under Sarbanes-Oxley related to accounting controls and procedures in the future, or, if we discover material weaknesses and other deficiencies in our internal control and accounting procedures, our stock price could decline significantly and raising capital could be more difficult. |
We caution you that the foregoing list may not contain all of the forward-looking statements made in this prospectus. We have based these forward-looking statements largely on our current expectations about future events and financial trends that we believe may affect our financial condition, results of operations, business strategy, short term and long-term business operations and objectives, and financial needs. These forward-looking statements are subject to a number of risks, uncertainties and assumptions, including those described in “Risk Factors”. Moreover, we operate in a very competitive and rapidly changing environment. New risks emerge from time to time. It is not possible for our management to predict all risks, nor can we assess the impact of all factors on our business or the extent to which any factor, or combination of factors, may cause actual results to differ materially from those contained in any forward-looking statements we may make. In light of these risks, uncertainties and assumptions, the forward-looking events and circumstances discussed in this prospectus may not occur and actual results could differ materially and adversely from those anticipated or implied in the forward-looking statements.
You should not rely upon forward-looking statements as predictions of future events. Although we believe that the expectations reflected in the forward-looking statements are reasonable, we cannot guarantee that the future results, levels of activity, performance or events and circumstances reflected in the forward-looking statements will be achieved or occur. We undertake no obligation to update publicly any forward-looking statements for any reason after the date of this prospectus to conform these statements to actual results or to changes in our expectations.
You should read this prospectus and the documents that we reference in this prospectus and have filed with the Securities and Exchange Commission as exhibits to the registration statement of which this prospectus is a part with the understanding that our actual future results, levels of activity, performance and events and circumstances may be materially different from what we expect.
We estimate that the net proceeds from this offering will be approximately $5,180,000 (or approximately $5,999,000 if the over-allotment option is exercised in full) after deducting the underwriter discount and estimated offering expenses payable by us.
We will only receive additional proceeds from the exercise of the Underwriter Warrants if such Warrants are exercised and the holders of such Warrants pay the exercise price in cash.
We currently intend to use the net proceeds of this offering for working capital and for general corporate purposes, including funding our research and development activities, consisting primarily of our ongoing clinical trials, and the legal, accounting and various other costs of being a public company. Subject to the availability of adequate operating capital, from this offering or from other sources, we also plan to expand our investor relations and public relations programs over the next twelve to eighteen months, at a cost of up to approximately $2,000,000, to improve communications with the financial community, create targeted profiles, expand media distribution, and build a digital community with an ongoing interest in our business prospects and activities.
The actual allocation of proceeds realized from this offering will depend upon our cash position and our working capital requirements. We cannot currently allocate specific percentages of the net proceeds to us from this offering that we may use for these purposes. Therefore, as of the date of this prospectus, we cannot specify with certainty all of the particular uses for the net proceeds to be received upon the completion of this offering. Accordingly, we will have discretion in the application of the net proceeds, and investors will be relying on our judgment regarding the application of the proceeds of this offering. Pending our use of the net proceeds from this offering, we intend to invest the net proceeds in a variety of capital preservation investments, including short-term, investment-grade, interest-bearing instruments and U.S. government securities.
| 26 |
The following table sets forth our capitalization as of March 31, 2025 as follows:
| ● | on an actual basis; | |
| ● | on an as adjusted basis, to reflect the conversion of 350,000 shares of Series A Convertible Preferred Stock outstanding into 72,917 shares of common stock pursuant to a notice of conversion dated May 16, 2025; and | |
| ● | On an as further adjusted basis, to reflect the issuance and sale of 5,263,158 shares of Common Stock in the offering as described herein at the public offering price of $1.14 per share of Common Stock, assuming no sale of Pre-Funded Warrants or exercise of the underwriter over-allotment option, after deducting underwriter fees and estimated offering expenses and the receipt of the proceeds of such sale. |
The information below is illustrative only. Our capitalization following the closing of this Common Stock offering will change based on the actual public offering price and other terms of this offering determined at pricing. You should read this table in conjunction with “Item 7. Management’s Discussion and Analysis of Financial Condition and Results of Operations” and the unaudited consolidated financial statements and related notes included in our Quarterly Report on Form 10-Q for the quarterly period ended March 31, 2025.
| As of March 31, 2025 | ||||||||||||
| Actual |
As Adjusted |
As Further Adjusted |
||||||||||
| (unaudited) | (unaudited) | (unaudited) | ||||||||||
| Cash | $ | 1,384,697 | $ | 1,384,697 | $ | 6,564,697 | ||||||
| Stockholders’ equity: | ||||||||||||
| Preferred stock, $0.0001 par value per share, 10,000,000 shares authorized; issued and outstanding – 350,000 shares | $ | 3,500,000 | $ | — | $ | — | ||||||
| Common stock, $0.0001 par value per share; 100,000,000 shares authorized; issued and outstanding – 2,684,074 shares actual, 2,756,991 shares on an as adjusted basis, and 8,020,149 shares on an as further adjusted basis | 268 | 275 | 802 | |||||||||
| Additional paid-in capital | 50,436,110 | 53,936,103 | 59,115,577 | |||||||||
| Accumulated deficit | (52,777,248 | ) | (52,777,248 | ) | (52,777,248 | ) | ||||||
| Total stockholders’ equity | 1,159,130 | 1,159,130 | 6,339,130 | |||||||||
| Total capitalization | $ | 1,159,130 | $ | 1,159,130 | $ | 6,339,130 | ||||||
The number of shares of our common stock outstanding as set forth above excludes:
| ● | 662,078 shares of common stock issuable upon the exercise of common stock options issued to members of management, consultants and directors at a weighted average exercise price of $11.526 per share; | |
| ● | 1,275,758 shares of common stock issuable upon exercise of outstanding common stock warrants at an average exercise price of $11.254 per share of common stock, including 434,784 shares of common stock issuable upon exercise of 434,784 common stock warrants exercisable at $2.29 per share issued in our February 13, 2025 offering, 32,609 shares of common stock issuable upon exercise of 32,609 common stock warrants exercisable at $3.1088 per share issued to the placement agent in our February 13, 2025 offering, and 149,700 shares of common stock issuable upon exercise of 149,700 publicly traded warrants at $57.00 per share of common stock through November 30, 2025; | |
| ● | 133,339 shares of common stock reserved for future grants pursuant to our 2020 Stock Incentive Plan, as amended (the “2020 Plan”); and | |
| ● | 263,158 shares of common stock issuable upon exercise of the Underwriter Warrants at an exercise price of $1.425 per share of common stock issuable to the underwriter in this offering, assuming that the underwriter over-allotment option is not exercised. |
| 27 |
Unless otherwise indicated, this prospectus also assumes no sale of Pre-Funded Warrants, no exercise of any of the Underwriter Warrants, and no exercise of the underwriter over-allotment option.
DILUTION
If you invest in our Common Stock in this offering, your investment will be immediately and substantially diluted to the extent of the difference between the public offering price per share of our common stock and the as adjusted net tangible book value per share of our common stock after giving effect to the offering.
Our net tangible book value as of March 31, 2025 was $1,159,130, or $0.42 per share. Net tangible book value per share represents our total tangible assets less total liabilities, divided by the number of shares of common stock outstanding.
As adjusted net tangible book value dilution per share of common stock to new investors represents the difference between the amount per share of Common Stock of common stock that is paid by investors in the offering and the net tangible book value per share of common stock immediately after completion of the offering. After giving effect to the offering and our sale of the Common Stock in the offering at an assumed public offering price of $1.14 per share of Common Stock, and after deduction of underwriter fees from gross proceeds raised in the offering and estimated offering expenses payable by us, our as adjusted net tangible book value as of March 31, 2025 would have been $6,339,130 or $0.79 per share of common stock. This represents an immediate increase in net tangible book value of $0.37 per share of common stock to existing stockholders and an immediate dilution in net tangible book value of $0.35 per share of common stock to investors in the offering, as illustrated in the following table, based on the shares of Common Stock outstanding as of March 31, 2025, as adjusted to reflect the conversion of 350,000 shares of Series A Convertible Preferred Stock outstanding into 72,917 shares of common stock pursuant to a notice of conversion dated May 16, 2025.
The information below is illustrative only and assumes the maximum offering amount of $6,000,000 is sold. The dilution caused by this offering will change based on the actual public offering amount and price and other terms of this offering determined at pricing. You should read this table in conjunction with “Item 7. Management’s Discussion and Analysis of Financial Condition and Results of Operations” and the unaudited consolidated financial statements and related notes included in our Quarterly Report on Form 10-Q for the quarterly period ended March 31, 2025.
| Assumed offering price per share of common stock | $ | 1.14 | ||||||
| Actual net tangible book value per share of common stock before this offering(1) | $ | 0.42 | ||||||
| Increase in net tangible book value per share attributable to new investors(2) | $ | 0.37 | ||||||
| Net tangible book value per share after this offering(3) | $ | 0.79 | ||||||
| Immediate dilution in net tangible book value per share to new investors | $ | 0.35 |
| (1) | Determined by dividing (i) net tangible book value (total assets less intangible assets) less total liabilities by (ii) the total number of shares of common stock issued and outstanding prior to the offering. |
| (2) | Represents the difference between (i) as adjusted net tangible book value per share after this offering and (ii) net tangible book value per share as of March 31, 2025. |
| (3) | Determined by dividing (i) as adjusted net tangible book value, which is our net tangible book value plus the cash proceeds of this offering, after deducting the estimated offering expenses payable by us, by (ii) the total number of shares of common stock to be outstanding following this offering. |
| 28 |
An increase of $0.25 in the assumed public offering price of $1.14 per share of Common Stock would increase the net tangible book value per share after this offering by $0.106 per share and increase the dilution to new investors purchasing Common Stock in this offering by $0.144 per share, assuming the amount of the offering as set forth on the cover page of this prospectus remains the same, and after deducting the underwriter fees and estimated offering expenses payable by us.
A decrease of $0.25 in the assumed public offering price of $1.14 per share of Common Stock would decrease the net tangible book value per share after this offering by $0.123 per share and would decrease the dilution to new investors purchasing Common Stock in this offering by $0.127 per share, assuming the amount of the offering as set forth on the cover page of this prospectus remains the same, and after deducting the underwriter fees and estimated offering expenses payable by us.
If we only sell 75%, 50% or 25% of the maximum offering amount of $6,000,000, our net tangible book value per share after this offering would be $0.742, $0.670 or $0.551, respectively, and the immediate dilution in net tangible book value per share to new investors purchasing Common Stock in this offering would be $0.398, $0.470 or $0.598, respectively, assuming no Pre-Funded Warrants are issued and after deducting the underwriter fees and estimated offering expenses payable by us.
If the underwriter exercises its over-allotment option in full to purchase an additional 789,143 shares of Common Stock at $1.14 per share, our as further adjusted net tangible book value, after giving effect to this offering, would have been approximately$0.813 per share, representing an increase in net tangible book value of approximately $0.393 per share to existing stockholders and immediate dilution in net tangible book value of approximately $0.327 per share to new investors purchasing shares in this offering.
The information discussed above is illustrative only and will adjust based on the actual public offering price, the actual number of shares of Common Stock that we offer in this offering, and other terms of this offering determined at the time of pricing. The foregoing discussion and table assume no issuance of Pre-Funded Warrants, which if sold, would reduce the number of shares of Common Stock that we are offering on a one-for-one basis. In addition, we may choose to raise additional capital due to market conditions or strategic considerations. To the extent that additional capital is raised through the sale of equity or convertible debt securities, the issuance of these securities could result in further dilution to our stockholders.
The number of shares of our common stock outstanding as set forth above excludes:
| ● | 662,078 shares of common stock issuable upon the exercise of common stock options issued to members of management, consultants and directors at a weighted average exercise price of $11.526 per share; | |
| ● | 1,275,758 shares of common stock issuable upon exercise of outstanding common stock warrants at an average exercise price of $11.254 per share of common stock, including 434,784 shares of common stock issuable upon exercise of 434,784 common stock warrants exercisable at $2.29 per share issued in our February 13, 2025 offering, 32,609 shares of common stock issuable upon exercise of 32,609 common stock warrants exercisable at $3.1088 per share issued to the placement agent in our February 13, 2025 offering, and 149,700 shares of common stock issuable upon exercise of 149,700 publicly traded warrants at $57.00 per share of common stock through November 30, 2025; | |
| ● | 133,339 shares of common stock reserved for future grants pursuant to our 2020 Stock Incentive Plan, as amended (the “2020 Plan”); and | |
| ● | 263,158 shares of common stock issuable upon exercise of common stock warrants at an exercise price of $1.425 per share of common stock issuable to the underwriter in this offering, assuming that the underwriter over-allotment option is not exercised. |
Unless otherwise indicated, this prospectus also assumes no sale of Pre-Funded Warrants, no exercise of any of the Underwriter Warrants, and no exercise of the underwriter over-allotment option.
| 29 |
SELECTED HISTORICAL FINANCIAL DATA
Consolidated Statements of Operations Data
| Three Months Ended March 31, | ||||||||
| 2025 | 2024 | |||||||
| Revenues | $ | — | $ | — | ||||
| Costs and expenses: | ||||||||
| Research and development costs | 91,457 | 119,064 | ||||||
| General and administrative costs | 615,483 | 847,815 | ||||||
| Total costs and expenses | 706,940 | 966,879 | ||||||
| Loss from operations | (706,940 | ) | (966,879 | ) | ||||
| Interest income | 441 | 2,859 | ||||||
| Interest expense | (3,135 | ) | (7,186 | ) | ||||
| Foreign currency gain (loss) | 79 | (116 | ) | |||||
| Net loss | $ | (709,555 | ) | $ | (971,322 | ) | ||
| Net loss per share of common stock – basic and diluted | $ | (0.29 | ) | $ | (0.43 | ) | ||
| Weighted average shares of common stock outstanding – basic and diluted | 2,471,513 | 2,249,290 | ||||||
Consolidated Balance Sheet Data
| March 31, | ||||||||
| 2025 | 2024 | |||||||
| Total current assets | $ | 1,514,228 | $ | 3,589,203 | ||||
| Total assets | $ | 1,514,228 | $ | 3,589,203 | ||||
| Total current liabilities | $ | 355,098 | $ | 462,836 | ||||
| Total liabilities | $ | 355,098 | $ | 462,836 | ||||
| Stockholders’ equity: | ||||||||
| Preferred Stock, $0.0001 par value; authorized – 10,000,000 shares; issued and outstanding – 350,000 shares of Series A Convertible Preferred Stock, $10.00 per share stated value, liquidation preference based on assumed conversion into common stock – 72,917 shares at March 31, 2025 and 2024 | $ | 3,500,000 | $ | 3,500,000 | ||||
| Common stock, $0.0001 par value; authorized – 100,000,000 shares; issued and outstanding – 2,684,074 shares and 2,249,290 shares at March 31, 2025 and 2024, respectively | 268 | 225 | ||||||
| Additional paid-in capital | 50,436,110 | 49,079,192 | ||||||
| Accumulated deficit | (52,777,248 | ) | (49,453,050 | ) | ||||
| Total stockholders’ equity | $ | 1,159,130 | $ | 3,126,367 | ||||
| 30 |
Consolidated Statements of Operations Data
| Years Ended December 31, | ||||||||
| 2024 | 2023 | |||||||
| Revenues | $ | — | $ | — | ||||
| Costs and expenses: | ||||||||
| Research and development costs | 726,232 | 898,100 | ||||||
| General and administrative costs | 2,846,557 | 4,192,136 | ||||||
| Total costs and expenses | 3,572,789 | 5,090,236 | ||||||
| Loss from operations | (3,572,789 | ) | (5,090,236 | ) | ||||
| Interest income | 7,048 | 17,486 | ||||||
| Interest expense | (16,821 | ) | (16,233 | ) | ||||
| Foreign currency gain (loss) | (3,403 | ) | 1,954 | |||||
| Net loss | $ | (3,585,965 | ) | $ | (5,087,029 | ) | ||
| Net loss per share of common stock – basic and diluted | $ | (1.59 | ) | $ | (2.66 | ) | ||
| Weighted average shares of common stock outstanding – basic and diluted | 2,249,290 | 1,915,838 | ||||||
Consolidated Balance Sheet Data
| December 31, | ||||||||
| 2024 | 2023 | |||||||
| Total current assets | $ | 1,145,503 | $ | 4,308,620 | ||||
| Total assets | $ | 1,145,503 | $ | 4,308,620 | ||||
| Total current liabilities | $ | 318,824 | $ | 313,858 | ||||
| Total liabilities | $ | 318,824 | $ | 313,858 | ||||
| Stockholders’ equity: | ||||||||
| Preferred Stock, $0.0001 par value; authorized – 10,000,000 shares; issued and outstanding – 350,000 shares of Series A Convertible Preferred Stock, $10.00 per share stated value, liquidation preference based on assumed conversion into common stock – 72,917 shares | $ | 3,500,000 | $ | 3,500,000 | ||||
| Common stock, $0.0001 par value; authorized – 100,000,000 shares; issued and outstanding – 2,249,290 shares at December 31, 2024 and 2023, respectively | 225 | 225 | ||||||
| Additional paid-in capital | 49,394,687 | 48,976,265 | ||||||
| Accumulated deficit | (52,067,693 | ) | (48,481,728 | ) | ||||
| Total stockholders’ equity | $ | 827,219 | $ | 3,994,762 | ||||
| 31 |
General
Our certificate of incorporation, as amended, authorizes the issuance of up to 100,000,000 shares of common stock, par value $0.0001 per share, and up to 10,000,000 shares of preferred stock, par value $0.0001 per share.
As of March 31, 2025, there were 2,684,074 shares of common stock outstanding, which were held by 46 stockholders of record, and 350,000 shares of Series A Convertible Preferred Stock outstanding, which were subsequently converted into 72,917 shares of common stock pursuant to a notice of conversion dated May 16, 2025.
On June 2, 2023, we effected a reverse stock split of our common stock at a ratio of 1-for-10. All share and per share information presented in this prospectus reflects the effect of the reverse stock split.
Common Stock
Each holder of common stock is entitled to one vote for each share of common stock held on all matters submitted to a vote of the stockholders, including the election of directors. Our certificate of incorporation, as amended and bylaws do not provide for cumulative voting rights.
Subject to preferences that may be applicable to any then outstanding preferred stock, the holders of our outstanding shares of common stock are entitled to receive dividends, if any, as may be declared from time to time by our Board of Directors out of legally available funds. In the event of our liquidation, dissolution or winding up, holders of common stock will be entitled to share ratably in the net assets legally available for distribution to stockholders after the payment of all of our debts and other liabilities, subject to the satisfaction of any liquidation preference granted to the holders of any outstanding shares of preferred stock.
Holders of our common stock have no pre-emptive, conversion or subscription rights, and there are no redemption or sinking fund provisions applicable to the common stock. The rights, preferences and privileges of the holders of common stock are subject to, and may be adversely affected by, the rights of the holders of shares of any series of our preferred stock that are outstanding or that we may designate and issue in the future.
Preferred Stock
Our Board of Directors is authorized, without vote or action by our stockholders, to issue from time to time up to an aggregate of 10,000,000 shares of preferred stock in one or more series and to fix or alter the designations, preferences, rights and any qualifications, limitations or restrictions of the shares of each of these series, including, if applicable, the dividend rights and preferences, conversion rights, voting rights, terms and rights of redemption, including without limitation sinking fund provisions, redemption price or prices, liquidation rights and preferences, and the number of shares constituting any series. The issuance of preferred stock may have the effect of delaying, deferring or preventing a change in control of us without further action by our stockholders and may adversely affect the dividend, liquidation and voting and other rights of the holders of common stock. The issuance of preferred stock with voting and conversion rights may adversely affect the voting power of the holders of common stock, including the loss of voting control to others. We currently have no plans to issue any additional shares of preferred stock.
We believe that the ability to issue preferred stock without the expense and delay of a special stockholders’ meeting provides us with increased flexibility in structuring possible future financings and acquisitions, and in meeting other corporate needs that might arise. This also permits the Board of Directors of the Company to issue preferred stock containing terms which could impede the completion of a takeover attempt. This could discourage an acquisition attempt or other transaction which stockholders might believe to be in their best interests or in which they might receive a premium for their stock over the then market price of the stock.
Warrants
In connection with the 2023 Financing and 2025 Financing, we issued warrants to purchase a total of 1,085,727 shares to the investors and placement agents, including Warrants to purchase 583,334 shares of common stock exercisable at $6.00 per share to the investor in the 2023 Financing and Warrants to purchase 434,784 shares of common stock exercisable at $2.29 per share to the investors in the 2025 Financing.
| 32 |
The exercise prices of the Warrants are subject to customary adjustments for stock splits, stock dividends, stock combinations, reclassifications, reorganizations, or similar events affecting our common stock. In addition, the Warrants contain a “fundamental transaction” provision which provides that if any defined fundamental transactions are within our control and are consummated, the holder of the unexercised common stock Warrants would be entitled to receive, at its option, in exchange for extinguishment of such Warrants, cash consideration equal to a Black-Scholes valuation amount, as defined in the Warrant agreement. The fundamental transaction provision includes (i) a sale, lease, assignment, transfer, conveyance or other disposition of all or substantially all of assets in one or a series of related transactions, or (ii) a change in control of the Company by which it, directly or indirectly, in one or more related transactions, consummates a stock or share purchase agreement or other business combination with another person or group, whereby such other person or group acquires more than 50% of the voting power of our common equity.
If such fundamental transaction is not within our control, including not being approved by our Board of Directors, the Warrant holder would only be entitled to receive the same type or form of consideration (and in the same proportion) equal to the Black-Scholes valuation amount of the remaining unexercised portion of the Warrant on the date of consummation of such fundamental transaction as the holders of our common stock receive. Accordingly, these Warrants are classified as a component of permanent stockholders’ equity. We will account for any cash payment for a Warrant redemption as a distribution from stockholders’ equity, as and when a fundamental transaction is consummated and such cash payment is made.
Anti-Takeover Effects of Certain Provisions in our Certificate and Bylaws
Exclusive Forum
The certificate of incorporation provides that, unless we consent to the selection of an alternative forum, the Court of Chancery of the State of Delaware shall be the sole and exclusive forum for (i) any derivative action or proceeding brought on our behalf, (ii) any action asserting a claim of breach of fiduciary duty owed by any of our directors, officers, or other employee to us or to our stockholders, (iii) any action asserting a claim against us arising pursuant to any provision of the Delaware General Corporation Law, the certificate of incorporation or the bylaws or (iv) any action asserting a claim governed by the internal affairs doctrine. However, this provision does not apply to suits brought to enforce a duty or liability created by the Exchange Act. In addition, the Court of Chancery of the State of Delaware and the federal district courts will have concurrent jurisdiction for the resolution of any suit brought to enforce any duty or liability created by the Securities Act. Notwithstanding the foregoing, the inclusion of such provisions in the certificate of incorporation will not be deemed to be a waiver by us or our stockholders of the obligation to comply with federal securities laws, rules and regulations.
Although we believe these provisions benefit the Company by providing increased consistency in the application of Delaware law in the types of lawsuits to which it applies, these provisions may have the effect of discouraging lawsuits against the Company’s directors and officers. Furthermore, the enforceability of choice of forum provisions in other companies’ certificates of incorporation has been challenged in legal proceedings, and it is possible that a court could find these types of provisions to be inapplicable or unenforceable.
Advance Notice of Stockholder Proposals and Nominations
Our bylaws include an advance notice procedure for stockholders to nominate candidates for election as directors or to bring other business before any meeting of our stockholders. The stockholder notice procedure provides that only persons who are nominated by, or at the direction of, the Board of Directors, or by a stockholder who has given timely written notice prior to the meeting at which directors are to be elected, will be eligible for election as directors and that, at a stockholders’ meeting, only such business may be conducted as has been brought before the meeting by, or at the direction of, the Board of Directors or by a stockholder who has given timely written notice of such stockholder’s intention to bring such business before such meeting.
| 33 |
Under the stockholder notice procedure, for notice of stockholder nominations or other business to be made at a stockholders’ meeting to be timely, such notice must be received by us not earlier than the close of business on the 120th calendar day and not later than the close of business on the 90th calendar day prior to the one-year anniversary of the immediately preceding year’s annual meeting or as otherwise provided in the bylaws.
A stockholder’s notice to us proposing to nominate a person for election as a director or proposing other business must contain certain information specified in the bylaws, including the identity and address of the nominating stockholder, a representation that the stockholder is a record holder of our stock entitled to vote at the meeting and information regarding each proposed nominee or each proposed matter of business that would be required under the federal securities laws to be included in a proxy statement soliciting proxies for the proposed nominee or the proposed matter of business.
The stockholder notice procedure may have the effect of precluding a contest for the election of directors or the consideration of stockholder proposals if the proper procedures are not followed, and of discouraging or deterring a third party from conducting a solicitation of proxies to elect its own slate of directors or to approve its own proposal, without regard to whether consideration of such nominees or proposals might be harmful or beneficial to us and our stockholder.
Restrictions on Call of Special Meetings
Our bylaws provide that special meetings of stockholders can only be called by the Board of Directors, Chief Executive Officer or President (in the absence of a Chief Executive Officer), but not by our stockholders or any other person or persons.
No Cumulative Voting
The certificate of incorporation does not authorize cumulative voting for the election of directors.
Preferred Stock Authorization
Our Board of Directors, without stockholder approval, has the authority under our certificate of incorporation to issue preferred stock with rights superior to the rights of the holders of common stock. As a result, preferred stock, while not intended as a defensive measure against takeovers, could be issued quickly and easily, could adversely affect the rights of holders of common stock, and could be issued with terms calculated to delay or prevent a change of control of the Company or make removal of management more difficult.
DESCRIPTION OF SECURITIES WE ARE OFFERING
Common Stock
The material terms and provisions of our common stock are described under the caption “Description of Securities”.
Pre-Funded Warrants
The following description of the Pre-Funded Warrants we are offering is a summary and is qualified in its entirety by reference to the provisions of the Pre-Funded Warrants, the form of which is filed as an exhibit to the registration statement of which this prospectus forms a part.
Each Pre-Funded Warrant is exercisable for one share of our common stock, with an exercise price equal to $0.00001 per share, at any time that the Pre-Funded Warrant is outstanding. There is no expiration date for the Pre-Funded Warrants. The holder of a Pre-Funded Warrant will not be deemed a holder of our underlying common stock until the Pre-Funded Warrant is exercised.
| 34 |
The exercise price and the number of shares of common stock issuable upon exercise of the Pre-Funded Warrants are subject to appropriate adjustment in the event of recapitalization events, stock dividends, stock splits, stock combinations, reclassifications, reorganizations or similar events affecting our common stock.
The term “pre-funded” refers to the fact that the purchase price of our common stock in this offering includes almost the entire exercise price that will be paid under the Pre-Funded Warrants, except for a nominal remaining exercise price of $0.00001. The purpose of the Pre-Funded Warrants is to enable investors that may have restrictions on their ability to beneficially own more than 4.99% (or, upon election of the holder, 9.99%) of our outstanding common stock following the consummation of this offering the opportunity to make an investment in us without triggering their ownership restrictions, by receiving Pre-Funded Warrants in lieu of our common stock which would result in such ownership of more than 4.99% (or 9.99%), and receive the ability to exercise their option to purchase the shares underlying the Pre-Funded Warrants at such nominal price at a later date.
The Pre-Funded Warrants are exercisable, at the option of the holder, in whole or in part, by delivering to us a duly executed exercise notice accompanied by payment in full for the number of shares of common stock purchased upon such exercise (except in the case of a cashless exercise, as discussed below). A holder (together with its affiliates) may not exercise any portion of the Pre-Funded Warrants to the extent that the holder would own more than 4.99% (or, at the election of the holder, 9.99%) of the outstanding shares of common stock immediately after exercise. However, upon notice from the holder to us, the holder may decrease or increase the holder’s beneficial ownership limitation, which may not exceed 9.99% of the number of outstanding shares of common stock immediately after giving effect to the exercise, as such percentage ownership is determined in accordance with the terms of the Pre-Funded Warrants, provided that any increase in the beneficial ownership limitation will not take effect until sixty-one (61) days following notice to us. Purchasers in this offering may also elect, prior to the issuance of the Pre-Funded Warrants, to have the initial exercise limitation set at 9.99% of our outstanding shares of common stock. No fractional shares will be issued in connection with the exercise of a Pre-Funded Warrant. In lieu of fractional shares, we will either pay the holder an amount in cash equal to the fractional amount multiplied by the exercise price or round up to the next whole share.
If at the time a holder exercises its Pre-Funded Warrants, a registration statement registering the issuance of the shares of common stock underlying the Pre-Funded Warrants under the Securities Act is not then effective or available and an exemption from registration under the Securities Act is not available for the issuance of such shares, then in lieu of making the cash payment otherwise contemplated to be made to us upon such exercise in payment of the aggregate exercise price, the holder may elect instead to receive upon such exercise (either in whole or in part) the net number of shares of common stock determined according to a formula set forth in the Pre-Funded Warrants.
Subject to applicable laws, a Pre-Funded Warrant may be transferred at the option of the holder upon surrender of the Pre-Funded Warrant to us together with the appropriate instruments of transfer.
Exchange Listing.
There is no trading market available for the Pre-Funded Warrants on any securities exchange or nationally recognized trading system. Accordingly, we do not intend to list the Pre-Funded Warrants on any securities exchange or nationally recognized trading system.
Right as a Stockholder.
Except as otherwise provided in the Pre-Funded Warrants or by virtue of such holder’s ownership of our shares of common stock, the holders of the Pre-Funded Warrants do not have the rights or privileges of holders of our shares of common stock, including any voting rights, until the holder exercises their Pre-Funded Warrants.
Fundamental Transaction.
In the event of a fundamental transaction, as described in the Pre-Funded Warrants and generally including any reorganization, recapitalization or reclassification of our shares of common stock, the sale, transfer or other disposition of all or substantially all of our properties or assets, our consolidation or merger with or into another person, the acquisition of more than 50% of our outstanding shares of common stock, or any person or group becoming the beneficial owner of more than 50% of the voting power represented by our outstanding shares of common stock, the holders of the Pre-Funded Warrants will be entitled to receive upon exercise of the Pre-Funded Warrants the kind and amount of securities, cash or other property that the holders would have received had they exercised the Pre-Funded Warrants immediately prior to such fundamental transaction.
| 35 |
We will enter into an underwriting agreement with Spartan Capital Securities, LLC in connection with this offering. Spartan is acting as the sole book-running manager. The underwriting agreement provides for the purchase of a specific number of shares of Common Stock. The underwriter has agreed to purchase the number of share of Common Stock set forth opposite its name below:
| Underwriter |
Number of Shares |
|||
| Spartan Capital Securities, LLC | 5,263,158 | |||
The underwriting agreement provides that the underwriter’s obligation to purchase Common Stock depends on the satisfaction of the conditions contained in the underwriting agreement including:
| ● | the representations and warranties made by us to the underwriter are true; | |
| ● | there is no material change in our business or the financial markets; and | |
| ● | we deliver customary closing documents to the underwriter. |
The underwriter has agreed to purchase all of the shares of Common Stock offered by this prospectus (other than those covered by the over-allotment option described below), if any are purchased under the underwriting agreement.
The underwriter is offering the Common Stock subject to various conditions and may reject all or part of any order. The underwriter has advised us that the underwriter proposes to offer the Common Stock directly to the public at the public offering price per share of Common Stock that appears on the cover page of this prospectus. In addition, the underwriter may offer some of the Common Stock to other securities dealers at such price less a concession of $0.04 per share of Common Stock. After the shares of Common Stock are released for sale to the public, the underwriter may change the offering price and other selling terms at various times.
We have granted the underwriter an over-allotment option to purchase up to 789,474 shares of Common Stock, representing 15% of the Common Stock sold in the offering at an assumed public offering price of $1.14 per share of Common Stock, solely to cover any over-allotments in connection with the sale of the Common Stock. The underwriter may exercise this over-allotment option in whole or in part at any time within forty-five (45) calendar days after the date of the offering.
If the underwriter exercises all or part of this option, Spartan will pay for Common Stock covered by the option at the public offering price that appears on the cover page of this prospectus, less the underwriter discount. The underwriter has agreed that, to the extent the over-allotment option is exercised, it will purchase the additional shares, reflected in the foregoing table.
We have also granted the underwriter five-year warrants to purchase up to 5.0% of share of Common Stock and the over-allotment option at an exercise price of 125% of the offering price per share of Common Stock.
| 36 |
The following table provides information regarding the amount of the discount to be paid to the underwriter by us, before expenses (these amounts are shown assuming both no exercise and full exercise of the underwriter’s over-allotment option in the offering):
|
Per Share |
Total Without Exercise of Underwriter’s Over-Allotment Option |
Total With Full Exercise of Underwriter’s Over-Allotment Option |
||||||||||
| Public offering price | $ | $ | $ | |||||||||
| Underwriter discount (8.0%) | $ | ( | ) | $ | ( | ) | $ | ( | ) | |||
| Non-accountable expense allowance (1.0%)(1) | $ | ( | ) | $ | ( | ) | $ | ( | ) | |||
| Proceeds to us (before expenses) | $ | $ | $ | |||||||||
(1) We have agreed to pay a non-accountable expense allowance to Spartan equal to 1.0% of the gross proceeds received in this offering. We have also agreed to reimburse Spartan for up to $125,000 of out-of-pocket fees and expenses, including, but not limited to, legal fees and disbursements for the underwriter’s counsel.
From time to time, Spartan or its affiliates have in the past or may in the future engage in investment banking and/or other services with us and our affiliates for which they have received or may in the future receive customary fees and expenses.
Lock-Up Agreements
Our directors, executive officers and 10% stockholders have agreed that, for a period of sixty (60) days from the closing date of the offering, subject to certain limited exceptions, they will not directly or indirectly, without the prior written consent of Spartan, (a) offer, sell, or otherwise transfer or dispose of, directly or indirectly, any shares of capital stock of the Company or any securities convertible into or exercisable or exchangeable for shares of capital stock of the Company; or (b) file or caused to be filed any registration statement with the Commission relating to the offering of any shares of capital stock of the Company or any securities convertible into or exercisable or exchangeable for shares of capital stock of the Company.
Spartan, in its sole discretion, may release the common stock and other securities subject to the lock-up agreements described above in whole or in part at any time. When determining whether or not to release common stock and other securities from lock-up agreements, Spartan will consider, among other factors, the holder’s reasons for requesting the release, the number of shares of common stock and other securities for which the release is being requested and market conditions at the time.
Company Standstill
We have agreed, for a period of sixty (60) days after the closing date of the offering (the “Standstill Period”), that without the prior written consent of Spartan, we will not (a) offer, sell, issue, or otherwise transfer or dispose of, directly or indirectly, any equity of our Company or any securities convertible into or exercisable or exchangeable for equity of our Company; (b) file or caused to be filed any registration statement with the Commission relating to the offering of any equity of our Company or any securities convertible into or exercisable or exchangeable for equity of our Company; or (c) enter into any agreement or announce the intention to effect any of the actions described in subsections (a) or (b) hereof (all of such matters, the “Standstill Restrictions”). So long as none of such equity securities shall be saleable in the public market until the expiration of the Standstill Period, the following matters shall not be prohibited by the Standstill Restrictions: (i) the adoption of an equity incentive plan and the grant of awards or equity pursuant to any equity incentive plan, and the filing of a registration statement on Form S-8; and (ii) securities issued pursuant to acquisitions or strategic transactions approved by a majority of the disinterested directors of our Company, provided that such securities are issued as “restricted securities” (as defined in Rule 144) and carry no registration rights that require or permit the filing of any registration statement in connection therewith during the Standstill Period, and provided that any such issuance shall only be to a person or entity (or to the equity holders of an entity) which is, itself or through its subsidiaries, an operating company or an owner of an asset in a business synergistic with the business of our Company and shall provide to our Company additional benefits in addition to the investment of funds, but shall not include a transaction in which our Company is issuing securities primarily for the purpose of raising capital or to an entity whose primary business is investing in securities. In no event should any equity transaction during the Standstill Period result in the sale of equity at an offering price to the public less than that of this offering.
| 37 |
Underwriter Warrants
As additional compensation to Spartan, upon consummation of the offering, we will issue to Spartan or its designees warrants to purchase an aggregate number of shares of common stock equal to 5.0% of the number of shares of Common Stock issued in the offering, at an exercise price per share equal to 125.0% of the public offering price (the “Underwriter Warrants”). The Underwriter Warrants and the underlying shares of common stock will not be sold, transferred, assigned, pledged, or hypothecated, or be the subject of any hedging, short sale, derivative, put, or call transaction that would result in the effective economic disposition of the Underwriter Warrants by any person for a period of one hundred eighty (180) Calendar Days beginning on the date of commencement of sales of the offering in compliance with FINRA Rule 5110(e)(1).
The Underwriter Warrants will be exercisable from the date that is six (6) months from the commencement of the sales of the offering, and will expire five (5) years from the commencement of sales of the offering in compliance with FINRA Rule 5110(g)(8)(A). In addition, such Underwriter Warrants shall be exercisable on a cash basis, provided that if a registration statement registering the Common Stock underlying the Underwriter Warrants is not effective, the Underwriter Warrants may be exercised on a cashless basis. Furthermore, (i) the Underwriter Warrants do not have more than one demand registration right at our Company’s expense in compliance with FINRA Rule 5110(g)(8)(B); (ii) the Underwriter Warrants do not have a demand registration right with a duration of more than five (5) years from the commencement of sales of the public offering in compliance with FINRA Rule 5110(g)(8)(C); (iii) the Underwriter Warrants do not have piggyback registration rights with a duration of more than seven (7) years from the commencement of sales of the public offering in compliance with FINRA Rule 5110(g)(8)(D); and (iv) the Underwriter Warrants have anti-dilution terms that are consistent with FINRA Rule 5110(g)(8)(E) and (F).
Right of First Refusal
During the Right of First Refusal Period and subject to any Senior Right of First Refusal (as defined below), if we or any of our subsidiaries (a) decides to dispose of or acquire business units or acquire any of its outstanding securities or make any exchange or tender offer or enter into a merger, consolidation or other business combination or any recapitalization, reorganization, restructuring or other similar transaction, including, without limitation, an extraordinary dividend or distributions or a spin-off or split-off, and the Company decides to retain a financial advisor for such transaction, Spartan (or any affiliate designated by Spartan) shall have the right to act as the Company’s exclusive financial advisor for any such transaction; or (b) decides to finance or refinance any indebtedness, Spartan (or any affiliate designated by Spartan) shall have the right to act as sole book-runner, sole manager, sole placement agent or sole agent with respect to such financing or refinancing; or (c) decides to raise funds by means of a public offering (including an at-the-market facility) or a private placement or any other capital raising financing of equity, equity-linked or debt securities, Spartan (or any affiliate designated by Spartan) shall have the right to act as sole book-running manager, sole underwriter or sole placement agent for such financing. The Company shall provide written notice to Spartan of any proposed transaction set forth in (a) – (c) above, including a detailed term sheet. Spartan (including through its affiliates) shall have ten (10) business days following receipt of such detailed term sheet to determine whether to waive its rights under the right of first refusal. If Spartan or one of its affiliates decides to accept any such engagement, the agreement governing such engagement will contain, among other things, provisions for customary fees and terms for transactions of similar size and nature, including indemnification, which are appropriate to such a transaction, provided, however, that after Spartan delivers such engagement letter, the Company may notify Spartan that it wishes to terminate the right of first refusal by paying a fee of $10,000, to be effective only upon receipt of such payment. The Right of First Refusal Period shall be the period beginning on the date hereof and ending on the date that is the earlier of (i) six (6) months after the expiration or earlier termination of any senior right of first refusal (“Senior Right of First Refusal”) existing on the date hereof with respect to any transactions described in (a) – (c) above, or (ii) the date that is the three (3) year anniversary of the commencement of sales in the Offering.
| 38 |
Notwithstanding the foregoing, the decision to accept our engagement shall be made by Spartan or one of its affiliates, by a written notice to us, within ten (10) Business Days after the receipt of our notification of financing needs, including a detailed term sheet. Spartan’s determination of whether in any case to exercise its right of first refusal will be strictly limited to the terms on such term sheet, and any waiver of such right of first refusal shall apply only to such specific terms. If Spartan waives its right of first refusal, any deviation from such terms shall void the waiver and require us to seek a new waiver from the right of first refusal.
Tail Financing
Spartan shall be entitled to compensation with respect to any public or private offering or other financing or capital raising transaction of any kind to the extent that such financing or capital is provided to us by funds whom Spartan had contacted during the engagement period or introduced to us during the engagement period, if such tail financing is consummated at any time within the twelve (12) month period following the closing of the offering or the expiration or termination of the letter of engagement, as amended between Spartan and us dated April 23, 2025, as may be further amended from time to time.
Stabilization
In accordance with Regulation M under the Exchange Act, the underwriter may engage in activities that stabilize, maintain or otherwise affect the price of our common stock, including short sales and purchases to cover positions created by short positions, stabilizing transactions, syndicate covering transactions, penalty bids and passive market making.
| ● | Short positions involve sales by the underwriter of shares of common stock in excess of the number of shares the underwriter is obligated to purchase, which creates a syndicate short position. The short position may be either a covered short position or a naked short position. In a covered short position, the number of shares involved in the sales made by the underwriter in excess of the number of shares they are obligated to purchase is not greater than the number of shares that they may purchase by exercising their option to purchase additional shares. In a naked short position, the number of shares involved is greater than the number of shares in their option to purchase additional shares. The underwriter may close out any short position by either exercising their option to purchase additional shares or purchasing shares in the open market. |
| ● | Stabilizing transactions permit bids to purchase the underlying security as long as the stabilizing bids do not exceed a specific maximum price. |
| ● | Syndicate covering transactions involve purchases of our shares of common stock in the open market after the distribution has been completed to cover syndicate short positions. In determining the source of shares to close out the short position, the underwriter will consider, among other things, the price of shares available for purchase in the open market as compared to the price at which they may purchase shares through the underwriter’s option to purchase additional shares. If the underwriter sells more shares than could be covered by the underwriter’s option to purchase additional shares, thereby creating a naked short position, the position can only be closed out by buying shares in the open market. A naked short position is more likely to be created if the underwriter is concerned that there could be downward pressure on the price of the shares in the open market after pricing that could adversely affect investors who purchase in the offering. |
| ● | Penalty bids permit the representative to reclaim a selling concession from a syndicate member when the shares of common stock originally sold by the syndicate member is purchased in a stabilizing or syndicate covering transaction to cover syndicate short positions. |
| ● | In passive market making, market makers in our common stock who are underwriters or prospective underwriters may, subject to limitations, make bids for or purchase our common stock until the time, if any, at which a stabilizing bid is made. |
| 39 |
These activities may have the effect of raising or maintaining the market price of our common stock or preventing or retarding a decline in the market price of our common stock. As a result of these activities, the price of our common stock may be higher than the price that might otherwise exist in the open market. These transactions may be effected on the Nasdaq Capital Market or otherwise and, if commenced, may be discontinued at any time.
Neither we nor the underwriter makes any representation or prediction as to the direction or magnitude of any effect that the transactions described above may have on the price of our common stock. In addition, neither we nor the underwriter makes any representation that Spartan will engage in these stabilizing transactions or that any transaction, once commenced, will not be discontinued without notice.
Discretionary Accounts
The underwriter has informed us that they do not expect to make sales to accounts over which they exercise discretionary authority in excess of five percent (5%) of the securities being offered in this offering.
Other Relationships
The underwriter is a full service financial institution engaged in various activities, which may include sales and trading, commercial and investment banking, advisory, investment management, investment research, principal investment, hedging, market making, brokerage and other financial and non-financial activities and services. The underwriter may in the future provide various investment banking, commercial banking and other financial services for us and our affiliates for which they may in the future receive customary fees.
In the ordinary course of its business activities, the underwriter and its affiliates, officers, directors and employees may purchase, sell or hold a broad array of investments and actively traded securities, derivatives, loans, commodities, currencies, credit default swaps and other financial instruments for their own account and for the accounts of their customers, and such investment and trading activities may involve or relate to assets, securities and/or instruments of the issuer (directly, as collateral securing other obligation or otherwise) publish or express independent research views in respect of such assets, securities or instruments and may at any time hold, or recommend to clients that they should acquire, long and/or short positions in such assets, securities and instruments.
Electronic Offer, Sale and Distribution of Shares
A prospectus in electronic format may be made available on the websites maintained by the underwriter, if any, participating in this offering and the underwriter participating in this offering may distribute prospectuses electronically. The underwriter may agree to allocate a number of shares of Common Stock and Pre-Funded Warrants for sale to its online brokerage account holders. Internet distributions will be allocated by the underwriter that will make internet distributions on the same basis as other allocations. Other than the prospectus in electronic format, the information on these websites is not part of, nor incorporated by reference into, this prospectus or the registration statement of which this prospectus forms a part, has not been approved or endorsed by us or the underwriter in its capacity as underwriter, and should not be relied upon by investors.
Offer Restrictions Outside the United States
Other than in the United States, no action has been taken by us or the underwriter that would permit a public offering of the securities offered by this prospectus in any jurisdiction where action for that purpose is required. The securities offered by this prospectus may not be offered or sold, directly or indirectly, nor may this prospectus or any other offering material or advertisements in connection with the offer and sale of any such securities be distributed or published in any jurisdiction, except under circumstances that will result in compliance with the applicable rules and regulations of that jurisdiction. Persons who come into possession of this prospectus are advised to inform themselves about and to observe any restrictions relating to the offering and the distribution of this prospectus. This prospectus does not constitute an offer to sell or a solicitation of an offer to buy any securities offered by this prospectus in any jurisdiction in which such an offer or a solicitation is unlawful.
| 40 |
Indemnification
We have agreed to indemnify the underwriter against certain liabilities, including liabilities under the Securities Act of 1933.
Rules of the Securities and Exchange Commission may limit the ability of the underwriter to bid for or purchase shares before the distribution of the shares is completed. However, the underwriter may engage in the following activities in accordance with the rules:
● Stabilizing transactions — The underwriter may make bids or purchases for the purpose of pegging, fixing or maintaining the price of the shares, so long as stabilizing bids do not exceed a specified maximum.
● Over-allotments and syndicate covering transactions — The underwriter may sell more shares of our common stock in connection with this offering than the number of shares that they have committed to purchase. This over-allotment creates a short position for the underwriter. This short sales position may involve either “covered” short sales or “naked” short sales. Covered short sales are short sales made in an amount not greater than the underwriter’s over-allotment option to purchase additional shares in this offering described above. The underwriter may close out any covered short position either by exercising their over-allotment option or by purchasing shares in the open market. To determine how they will close the covered short position, the underwriter will consider, among other things, the price of shares available for purchase in the open market, as compared to the price at which they may purchase shares through the over-allotment option. Naked short sales are short sales in excess of the over-allotment option. The underwriter must close out any naked short position by purchasing shares in the open market. A naked short position is more likely to be created if the underwriter is concerned that, in the open market after pricing, there may be downward pressure on the price of the shares that could adversely affect investors who purchase shares in this offering.
● Penalty bids — If the representative purchases shares in the open market in a stabilizing transaction or syndicate covering transaction, it may reclaim a selling concession from the underwriter and selling group members who sold those shares as part of this offering.
● Passive market making — Market makers in the shares who is the underwriter may make bids for or purchases of shares, subject to limitations, until the time, if ever, at which a stabilizing bid is made.
Similar to other purchase transactions, the underwriter’s purchases to cover the syndicate short sales or to stabilize the market price of our common stock may have the effect of raising or maintaining the market price of our common stock or preventing or mitigating a decline in the market price of our common stock. As a result, the price of the shares of our common stock may be higher than the price that might otherwise exist in the open market. The imposition of a penalty bid might also have an effect on the price of the shares if it discourages resales of the shares.
Neither we nor the underwriter make any representation or prediction as to the effect that the transactions described above may have on the price of the shares. These transactions may occur on the Nasdaq Capital Market or otherwise. If such transactions are commenced, they may be discontinued without notice at any time.
● Electronic Delivery of Preliminary Prospectus — A preliminary prospectus in electronic format may be delivered to potential investors by one or more of the underwriters participating in this offering. The preliminary prospectus supplement in electronic format will be identical to the paper version of such preliminary prospectus. Other than the preliminary prospectus in electronic format, the information on any underwriter’s website and any information contained in any other website maintained by an underwriter is not part of this prospectus supplement, the accompanying prospectus or the registration statement of which this prospectus forms a part.
The underwriter and its affiliates may provide from time to time in the future certain commercial banking, financial advisory, investment banking and other services for us and our affiliates in the ordinary course of their business, for which they may receive customary fees and commissions. In addition, from time to time, the underwriter and its affiliates may effect transactions for their own account or the accounts of customers, and hold on behalf of itself or its customers, long or short positions in our debt or equity securities or loans, and may do so in the future. Spartan may release, or authorize us to release, as the case may be, the common stock and other securities subject to the lock-up agreements described above in whole or in part at any time with or without notice.
Listing
Our common stock is currently listed on the Nasdaq Capital Market under the symbol “LIXT”. The warrants issued in our November 2020 public offering are currently listed on the Nasdaq Capital Market under the symbol “LIXTW”.
Transfer Agent and Registrar
The transfer agent and registrar for our common stock and the warrants issued in our November 2020 public offering is Computershare Trust Company, N.A.
| 41 |
The validity of the securities offered by this prospectus will be passed upon by TroyGould PC, Los Angeles, California. Kaufman & Canoles, Richmond, Virginia is acting as counsel to the underwriter.
Weinberg & Company, P.A., our independent, registered public accounting firm, has audited our consolidated financial statements as of December 31, 2024 and 2023 and for the years then ended included in our Annual Report on Form 10-K for the fiscal year ended December 31, 2024, which is incorporated by reference into this prospectus and elsewhere in the registration statement of which this prospectus is a part. Our consolidated financial statements are incorporated by reference in reliance on Weinberg & Company P.A.’s report, which includes an explanatory paragraph regarding substantial doubt about the Company’s ability to continue as a going concern, given on their authority as experts in accounting and auditing.
INCORPORATION OF CERTAIN INFORMATION BY REFERENCE
The SEC allows us to incorporate by reference into this prospectus certain information we file with it, which means that we can disclose important information by referring you to those documents. The information incorporated by reference is considered to be part of this prospectus. Because we are incorporating by reference future filings with the SEC, this prospectus is continually updated and those future filings may modify or supersede some of the information included or incorporated in this prospectus. We incorporate by reference the documents listed below and all documents subsequently filed with the SEC (excluding any portions of any Form 8-K that are not deemed “filed” pursuant to the General Instructions of Form 8-K) pursuant to Section 13(a), 13(c), 14 or 15(d) of the Exchange Act, after the date of this prospectus and prior to the date this offering is terminated or we issue all of the securities under this prospectus:
| ● | our Annual Report on Form 10-K for the fiscal year ended December 31, 2024, filed with the SEC on March 24, 2025; | |
| ● | our Quarterly Report on Form 10-Q for the quarterly period ended March 31, 2025, filed with the SEC on May 12, 2025; | |
| ● | our Current Reports on Form 8-K, filed with the SEC on January 6, 2025, February 13, 2025, February 21, 2025, February 25, 2025, March 11, 2025, March 11, 2025, March 14, 2025, March 27, 2025, March 31, 2025, April 18, 2025, May 20, 2025, and June 17, 2025; and | |
| ● | the description of our common stock contained in the registration statement on Form 8-A, filed with the SEC on November 17, 2020, and any amendment or report filed for the purpose of updating such description (including Exhibit 4.1 to the Annual Report on Form 10-K for the fiscal year ended December 31, 2023). |
To obtain copies of these filings, see “Where You Can Find More Information” in this prospectus. Nothing in this prospectus shall be deemed to incorporate information furnished, but not filed, with the SEC, including pursuant to Item 2.02 or Item 7.01 of Form 8-K and any corresponding information or exhibit furnished under Item 9.01 of Form 8-K.
Information in this prospectus supersedes related information in the documents listed above and information in subsequently filed documents supersedes related information in both this prospectus and the incorporated documents.
WHERE YOU CAN FIND MORE INFORMATION
We are subject to the periodic reporting requirements of the Exchange Act, and we will file periodic reports, proxy statements and other information with the SEC. These periodic reports, proxy statements and other information are available at www.sec.gov. We maintain a website at https://lixte.com. We have not incorporated by reference into this prospectus the information contained in, or that can be accessed through, our website, and you should not consider it to be a part of this prospectus. You may access our Annual Reports on Form 10-K, Quarterly Reports on Form 10-Q, Current Reports on Form 8-K and amendments to those reports filed or furnished pursuant to Section 13(a) or 15(d) of the Exchange Act with the SEC free of charge at our website as soon as reasonably practicable after such material is electronically filed with, or furnished to, the SEC. You may also request a copy of these filings (other than exhibits to these documents unless the exhibits are specifically incorporated by reference into these documents or referred to in this prospectus), at no cost, by writing us at 680 East Colorado Boulevard, Suite 180, Pasadena, California 91101 or contacting us at (631) 830-7092.
We have filed with the SEC a registration statement under the Securities Act relating to the offering of these securities. The registration statement, including the attached exhibits, contains additional relevant information about us and the securities. This prospectus does not contain all of the information set forth in the registration statement. You may review a copy of the registration statement and the documents incorporated by reference herein through the SEC’s website at www.sec.gov.
| 42 |
LIXTE BIOTECHNOLOGY HOLDINGS, INC.
PROSPECTUS
June 17, 2025
PART II
INFORMATION NOT REQUIRED IN PROSPECTUS
Item 13. Other Expenses of Issuance and Distribution
The following table sets forth the various expenses, all of which will be borne by the registrant, in connection with the sale and distribution of the securities being registered, other than the underwriter fees. All amounts shown are estimates except for the SEC registration fee and the FINRA filing fee.
| SEC registration fee | $ | 918.60 | ||
| FINRA fees | 2,000.00 | |||
| Transfer agent and registrar fees | 10,000.00 | |||
| Accounting fees and expenses | 40,000.00 | |||
| Legal fees and expenses | 75,000.00 | |||
| Underwriter expense allowance | 125,000.00 | |||
| Miscellaneous | 27,081.40 | |||
| Total | $ | 280,000.00 |
Item 14. Indemnification of Directors and Officers.
Section 102(b)(7) of the Delaware General Corporation Law (“DGCL”) provides that a Delaware corporation, in its certificate of incorporation, may limit the personal liability of a director to the corporation or its stockholders for monetary damages for breach of fiduciary duties as a director, except for liability for any:
| ● | transaction from which the director derived an improper personal benefit; | |
| ● | act or omission not in good faith or that involved intentional misconduct or a knowing violation of law; | |
| ● | unlawful payment of dividends or redemption of shares; or | |
| ● | breach of the director’s duty of loyalty to the corporation or its stockholders. |
Under Section 145 of the DGCL, we can indemnify our directors and officers against liabilities they may incur in such capacities, including liabilities under the Securities Act of 1933, as amended (the “Securities Act”). Our certificate of incorporation (Exhibit 3.1 to this registration statement) provides that we must indemnify our directors and officers to the fullest extent permitted by law and requires us to pay expenses incurred in defending or other participating in any proceeding in advance of its final disposition upon our receipt of an undertaking by the director or officer to repay such advances if it is ultimately determined that the director or officer is not entitled to indemnification. Our certificate of incorporation further provides that rights conferred under such certificate of incorporation do not exclude any other right such persons may have or acquire under the certificate of incorporation, the bylaws, any statute, agreement, vote of stockholders or disinterested directors or otherwise.
The certificate of incorporation also provides that, pursuant to Delaware law, our directors shall not be liable for monetary damages for breach of the directors’ fiduciary duty of care to us and our stockholders. This provision in the certificate of incorporation does not eliminate the duty of care, and in appropriate circumstances equitable remedies such as injunctive or other forms of non-monetary relief will remain available under Delaware law. In addition, each director will continue to be subject to liability for breach of the director’s duty of loyalty to us for acts or omissions not in good faith or involving intentional misconduct, or knowing violations of law, for actions leading to improper personal benefit to the director, and for payment of dividends or approval of stock repurchases or redemptions that are unlawful under Delaware law. The provision also does not affect a director’s responsibilities under any other law, such as the federal securities laws or state or federal environmental laws. We also intend to obtain directors’ and officers’ liability insurance pursuant to which our directors and officers are insured against liability for actions taken in their capacities as directors and officers.
In addition, we have entered into agreements to indemnify our directors and certain of our officers in addition to the indemnification provided for in the certificate of incorporation. These agreements, among other things, indemnify our directors and some of our officers for certain expenses (including attorney’s fees), judgments, fines and settlement amounts incurred by such person in any action or proceeding, including any action by or in our right, on account of services by that person as a director or officer of our company or as a director or officer of our subsidiary, or as a director or officer of any other company or enterprise that the person provides services to at our request.
| II-1 |
Item 15. Recent Sales of Unregistered Securities.
The information below lists all of the securities sold by us during the past three years which were not registered under the Securities Act:
On May 16, 2025, the Company received a notice of conversion with respect to its 350,000 shares of Series A Convertible Preferred Stock outstanding, These shares of preferred stock were issued to an investor in 2015 and 2016 and were convertible into 72,917 shares of common stock on such date.
Item 16. Exhibits and Financial Statement Schedules.
A list of exhibits to this registration statement is set forth in the Index to Exhibits as presented below.
INDEX TO EXHIBITS
| II-2 |
| II-3 |
| II-4 |
| * | Filed herewith. |
| + | Indicates a management contract or any compensatory plan, contract or arrangement. |
| II-5 |
Item 17. Undertakings.
The undersigned registrant hereby undertakes:
| (a)(1) | To file, during any period in which offers or sales are being made, a post-effective amendment to this registration statement: |
| (i) | To include any prospectus required by section 10(a)(3) of the Securities Act of 1933; | |
| (ii) | To reflect in the prospectus any facts or events arising after the effective date of the registration statement (or the most recent post-effective amendment thereof) which, individually or in the aggregate, represent a fundamental change in the information set forth in the registration statement. Notwithstanding the foregoing, any increase or decrease in volume of securities offered (if the total dollar value of securities offered would not exceed that which was registered) and any deviation from the low or high end of the estimated maximum offering amount may be reflected in the form of prospectus filed with the SEC pursuant to Rule 424(b) if, in the aggregate, the changes in volume and price represent no more than 20% change in the maximum aggregate offering price set forth in the “Calculation of Registration Fee” table in the effective registration statement; and | |
| (iii) | To include any material information with respect to the plan of distribution not previously disclosed in the registration statement or any material change to such information in the registration statement; |
provided, however, that the undertakings set forth in paragraphs (a)(1)(i), (a)(1)(ii) and (a)(1)(iii) above do not apply if the information required to be included in a post-effective amendment by those paragraphs is contained in reports filed with or furnished to the SEC by the registrant pursuant to section 13 or section 15(d) of the Securities Exchange Act of 1934 that are incorporated by reference in this registration statement or are contained in a form of prospectus filed pursuant to Rule 424(b) that is part of this registration statement.
| (2) | That, for the purposes of determining any liability under the Securities Act of 1933, each post-effective amendment that contains a form of prospectus shall be deemed to be a new registration statement relating to the securities offered therein, and the offering of such securities at that time shall be deemed to be the initial bona fide offering thereof. | |
| (3) | To remove from registration by means of a post-effective amendment any of the securities being registered which remain unsold at the termination of the offering. | |
| (4) | That in a primary offering of securities of the undersigned registrant pursuant to this registration statement, regardless of the underwriting method used to sell the securities to the purchaser, if the securities are offered or sold to such purchaser by means of any of the following communications, the undersigned registrant will be a seller to the purchaser and will be considered to offer or sell such securities to such purchaser: |
| (i) | Any preliminary prospectus or prospectus of the undersigned registrant relating to the offering required to be filed pursuant to Rule 424; |
| II-6 |
| (ii) | Any free writing prospectus relating to the offering prepared by or on behalf of the undersigned registrant or used or referred to by the undersigned registrant; | |
| (iii) | The portion of any other free writing prospectus relating to the offering containing material information about the undersigned registrant or its securities provided by or on behalf of the undersigned registrant; and | |
| (iv) | Any other communication that is an offer in the offering made by the undersigned registrant to the purchaser. |
| (b) | The undersigned registrant hereby undertakes that, for the purposes of determining any liability under the Securities Act of 1933, each filing of the registrant’s annual report pursuant to Section 13(a) or 15(d) of the Securities Exchange Act of 1934 (and, where applicable, each filing of an employee benefit plan’s annual report pursuant to Section 15(d) of the Securities Exchange Act of 1934) that is incorporated by reference in the registration statement shall be deemed to be a new registration statement relating to the securities offered therein, and the offering of such securities at that time shall be deemed to be the initial bona fide offering thereof. | |
| (c) | Insofar as indemnification for liabilities arising under the Securities Act of 1933 may be permitted to directors, officers and controlling persons of the registrant pursuant to the foregoing provisions, or otherwise, the registrant has been advised that in the opinion of the Securities and Exchange Commission such indemnification is against public policy as expressed in the Act and is, therefore, unenforceable. In the event that a claim for indemnification against such liabilities (other than the payment by the registrant of expenses incurred or paid by a director, officer or controlling person of the registrant in the successful defense of any action, suit or proceeding) is asserted by such director, officer or controlling person in connection with the securities being registered, the registrant will, unless in the opinion of its counsel the matter has been settled by controlling precedent, submit to a court of appropriate jurisdiction the question whether such indemnification by it is against public policy as expressed in the Act and will be governed by the final adjudication of such issue. | |
| (d) | That, |
| (1) | For purposes of determining any liability under the Securities Act of 1933, the information omitted from the form of prospectus filed as part of this registration statement in reliance upon Rule 430A and contained in a form of prospectus filed by the registrant pursuant to Rule 424(b)(1) or (4) or 497(h) under the Securities Act of 1933 shall be deemed to be part of this registration statement as of the time it was declared effective. | |
| (2) | For the purpose of determining any liability under the Securities Act of 1933, each post-effective amendment that contains a form of prospectus shall be deemed to be a new registration statement relating to the securities offered therein, and the offering of such securities at that time shall be deemed to be the initial bona fide offering thereof. |
| II-7 |
SIGNATURES
Pursuant to the requirements of the Securities Act of 1933, the registrant has duly caused this registration statement to be signed on its behalf by the undersigned, thereunto duly authorized, in the city of Pasadena, State of California, on June 17, 2025.
| Lixte Biotechnology Holdings, Inc. | ||
| By: | /s/ Geordan Pursglove | |
| Name: | Geordan Pursglove | |
| Title: | Chief Executive Officer | |
| (Principal Executive Officer) | ||
Each person whose signature appears below appoints Geordan Pursglove and Robert Weingarten, and each of them, each of whom may act without the joinder of the other, as their true and lawful attorney-in-fact and agent, with full power of substitution and resubstitution for them and in their name, place and stead, in any and all capacities to sign any and all amendments (including post-effective amendments) to this registration statement (and to any registration statement filed pursuant to Rule 462 under the Securities Act of 1933, as amended), and to file the same, with all exhibits thereto, and other documents in connection therewith, with the Securities and Exchange Commission, granting unto said attorneys-in-fact and agents full power and authority to do and perform each and every act and thing requisite and necessary to be done, as fully to all intents and purposes as they might or would do in person, hereby ratifying and confirming all that said attorneys-in-fact and agents or any of them or their substitute or substitutes, may lawfully do or cause to be done by virtue hereof.
Pursuant to the requirements of the Securities Act of 1933, this registration statement has been signed by the following persons in the capacities and on the dates indicated.
| Signature | Title(s) | Date | ||
| /s/ Geordan Pursglove | Chief Executive Officer and Chairman of the Board of Directors | June 17, 2025 | ||
| Geordan Pursglove | (Principal Executive Officer) | |||
| /s/ Bastiaan van der Baan | President, Chief Scientific Officer and Director | June 17, 2025 | ||
| Bastiaan van der Baan | ||||
| /s/ Robert Weingarten | Vice President and Chief Financial Officer | June 17, 2025 | ||
| Robert Weingarten | (Principal Financial and Accounting Officer) | |||
| * | Director | June 17, 2025 | ||
| Stephen Forman | ||||
| * | Director | June 17, 2025 | ||
| Yun Yen | ||||
| * | Director | June 17, 2025 | ||
| Rene Bernards | ||||
| * | Director | June 17, 2025 | ||
| Regina Brown |
*By: /s/ Robert Weingarten, Attorney-in-Fact
| II-8 |