EX-10.3
Published on August 7, 2025
Exhibit 10.3
Agreement for GSK & LIXTE Supported Collaborative Study
| Institution: | The University of Texas M. D. Anderson Cancer Center | |
| Investigator: | Amir Jazaeri, MD | |
| GSK Investigational Product: | Dostarlimab | |
| LIXTE Investigational Product: | LB-100 | |
| Protocol Number and Title: | 219582 “Safety and Efficacy of Targeting PP2A in Ovarian Clear Cell Carcinoma (OCCC) using Dostarlimab and LB-100” | |
| Effective Date: | Last Signature Date |
THIS SUPPORTED COLLABORATIVE STUDY AGREEMENT (together with Appendices A, B, C, D, E, F and G (collectively “Agreement”) is made as of the Effective Date by and between GlaxoSmithKline LLC, with an office at 1000 Winter Street, Suite 3300, Waltham, MA 02451 (“GSK”), Lixte Biotechnology Holdings, Inc., having offices at 680 E. Colorado Boulevard, Suite 180, Pasadena, CA 91101 (“LIXTE”), and The University of Texas M. D. Anderson Cancer Center, a government agency of the State of Texas and a member institution of the University of Texas System (“System”) having an address at 1515 Holcombe Blvd, Houston, TX., 77030 (“Institution”) GSK, LIXTE and Institution collectively referred to as “Parties” or in the singular “Party,” under the following terms and conditions.
| 1. | Background. Dr. Amir Jazaeri, MD (“Investigator”), who is employed by the Institution, wishes to conduct at Institution and at Participating Site (as defined in Section 7.3), a clinical study of GSK’s proprietary drug known as Dostarlimab provided by GSK hereunder (the “GSK Investigational Product”) and LIXTE’s proprietary drug known as LB-100 provided by LIXTE hereunder (the “LIXTE Investigational Product”) each provided at no charge to Institution as necessary for the conduct of the clinical study , (collectively the “Investigational Products”) under the protocol number and title specified above (as may be amended from time to time, the “Protocol”) (such clinical study is hereinafter referred to as the “Study”). The Parties are interested in the development of scientific and medical knowledge concerning the Investigational Products and ovarian cancer, and GSK is therefore willing to supply Institution with quantities of the GSK Investigational Product as specified in Appendix A, and as outlined in Schedule 1, Appendix A and LIXTE is willing to supply Institution with the quantities of the LIXTE Investigational Product, as specified in Appendix A (“Delineation of GSK and LIXTE Investigational Product Responsibilities”). GSK is willing to provide funding to support the Study as specified in Section 3.4 below and further described in Appendix B (the “Funding”), however, the Parties agree and acknowledge LIXTE is not providing any funding or compensation to Institution or GSK for conducting the Study under this Agreement. Institution agrees to conduct the Study under the terms and conditions set forth in this Agreement. Insitution is the regulatory sponsor of the Study and is responsible for all sponsor and investigator regulatory obligations for the Study, if and to the extent applicable to the Study and if the Study is not exempt from such regulation. The Parties have identified responsibilities for this Study as indicated in Appendix C (“Task Responsibility Matrix”). Institution will notify GSK and LIXTE promptly in writing of any proposed change of the Investigator. Given that the Investigator is not a legal party to this Agreement, all references in this Agreement that purport to or arguably impose a legal obligation on the Investigator will be deemed to be a legal obligation on Institution, and Institution will be responsible for ensuring that the Investigator complies with such obligation. |
| Page 1 of 38 Confidential |
| 2. | Compliance with Protocol/Law. |
| 2.1. | Institution will conduct the Study in accordance with (a) the Protocol (attached as Appendix D and acknowledged by the Parties as the confidential information of Institution); (b) this Agreement; (c) all applicable provisions of any and all federal, state and local laws, rules, regulations, orders and guidance relevant to the conduct of the Study including, (i) the United States Federal Food, Drug, and Cosmetic Act, as amended, and the applicable regulations promulgated under it from time to time, the Public Health Service Act, the Anti-Kickback Statute set forth at 42 U.S.C. § 1320a-7b(b), United States Code of Federal Regulations and applicable comparable state laws and regulations; (ii) the United States Health Insurance Portability and Accountability Act of 1996, as amended (“HIPAA”) and applicable comparable state laws and regulations; and (iii) publications of the International Conference on Harmonization of Technical Requirements for Registration of Pharmaceuticals for Human Use as adopted by the United States Food and Drug Administration (“FDA”), including current Good Clinical Practice (GCP) guidelines. Institution will be the regulatory sponsor of the Study and will fulfill, or cause the fulfillment of, all responsibilities of a regulatory sponsor (including postings on clinicaltrials.gov), if applicable to the Study and if the Study is not exempt from such regulation. If necessary, the Institution has filed or will file, and will maintain, an Investigational New Drug Application (“IND”) authorizing the Study with the FDA. Institution shall obtain and maintain, or shall cause Investigator to obtain and maintain, all other required authorizations for, and reviews of, the Study including approval of the IRB (as defined in Section 5 below) and proper oversight by all other applicable entities (e.g., ethics committees). Institution represents that the Investigator has the necessary licenses and has the expertise, time and resources to perform this Study. |
| 2.2. | Should there be any inconsistency between the Protocol and the terms of this Agreement, the language of this Agreement shall control over the Protocol or any amendments thereto, except that the terms of the Protocol (or any amendments thereto) shall govern with respect to medical, scientific and clinical issues of the Study. |
| 2.3. | The Parties will use reasonable efforts to conduct the Study in adherence with the Anticipated Timelines set out in Appendix E (“Anticipated Timelines”). Any material change, deviation, delay, modification, or reasonably expected delay to the Anticipated Timelines of which a Party becomes aware will be documented in writing to the other Parties for awareness and possible resolution. |
| 3. | GSK & LIXTE Support |
| 3.1. | Investigational Products Supply and Accountability. GSK and LIXTE each agree to provide Institution and Investigator with the quantities of GSK Investigational Product and LIXTE Investigational Product, respectively, as specified in Appendix A. For the avoidance of doubt, Institution and Investigator are performing the Study independently of GSK and LIXTE, and GSK and LIXTE have not determined the design of the Protocol and are not responsible for its implementation. Institution or its authorized designee will: (a) verify receipt of the GSK and LIXTE Investigational Products by signing the appropriate documentation provided by GSK and LIXTE or their respective designee; (b) handle and store all Investigational Products securely and in compliance with all applicable laws, rules and regulations and as specified in the Protocol, the applicable Investigational Products labeling and/or in writing by GSK or LIXTE, as the case may be; (c) be responsible for any additional labeling (e.g., for dispensing the Investigational Products for the Study) in compliance with and as required by applicable laws and the Protocol; (d) maintain complete and accurate records of use and disposition of the Investigational Products; (e) only dispense, or permit to be dispensed, the GSK Investigational Product and LIXTE Investigational Product supplied under this Agreement to Study subjects in accordance with the Protocol; and (f) after completion or early termination of the Study, at GSK’s or LIXTE’s respective expense, (i) return to GSK (or its designee) or destroy (as instructed by GSK) all unused GSK Investigational Product in accordance with the Protocol, applicable laws and regulations, and any reasonable written instructions provided by GSK to Institution, and, in the event of destruction of GSK Investigational Product, provide GSK, upon request, with a certificate/documentation of destruction to GSK’s reasonable satisfaction, and (ii) return to LIXTE (or its designee) or destroy (as instructed by LIXTE) all unused LIXTE Investigational Product in accordance with the Protocol, applicable laws and regulations, and any reasonable written instructions provided by LIXTE to Institution, and, in the event of destruction of LIXTE Investigational Product, provide LIXTE, upon request, with a certificate/documentation of destruction to LIXTE’s reasonable satisfaction. Notwithstanding the foregoing, Institution will have the right to destroy expired Investigational Products after thirty (30) days of the Investigational Products’ respective expiration date, unless otherwise agreed to by the respective Parties. Additionally, Institution and Investigator shall ensure that any Study subject clinical samples collected for purposes of the Study as required by the Protocol to be tested during and in the course of the Study are tested in accordance with the Protocol or are otherwise taken in accordance with standard of care. Investigational Products shall, at all times remain solely under the responsibility and overall control of the Institution until used in the Study, returned or destroyed. Institution and Investigator shall not supply or permit any other person to supply the Investigational Products to any third party, that is, any person who is not participating in the Study as a member of the Study Personnel (defined in Section 7.2 below) or a subject enrolled in the Study. GSK and LIXTE shall supply the GSK and LIXTE Investigational Product only after and as long as the Institution has obtained and holds the necessary authorizations and/or approvals for the Study in accordance with Section 5 below. |
| Page 2 of 38 Confidential |
(a) Neither GSK nor LIXTE will be obligated to provide any quantity of the Investigational Products and/or Funding other than as specified in Appendix A and Appendix B, respectively, unless such obligation to provide additional Investigational Products and/or Funding is included in a written amendment to this Agreement signed by Institution, GSK and LIXTE.
(b) GSK & LIXTE will provide Institution and/or Investigator label proofs of such Investigational Products shipments for review, which are in compliance to FDA criteria, and understand that all Investigational Products should be shipped to Institution in accordance with Appendix G, provided that GSK and LIXTE are provided written details of shipping details for the Participating Sites from the Institution and/or Investigator so that GSK and LIXTE can ship Investigational Products directly to Participating Sites.
| 3.2. | Institution will not, and will ensure Investigator does not, and Institution will not (and will ensure Investigator does not) permit any third parties to, modify or reverse engineer, combine with another material, or formulate the Investigational Products or use the Investigational Products, in each case other than specified in this Agreement or the applicable Protocol without GSK’s and LIXTE’s prior written agreement for their respective Investigational Product. |
| 3.3. | Misconduct. If GSK or LIXTE reasonably believes with sufficient evidence there has been any research misconduct in relation to the Study, GSK or LIXTE will inform Institution and the Investigator and Institution will reasonably cooperate, and will cause Investigator to reasonably cooperate, to conduct a thorough investigation into any alleged research and, to the extent permitted by law, provide GSK or LIXTE with any applicable findings and/or outcomes. |
| 3.4. | Funding. GSK agrees to provide the Funding to Institution for the Study as set forth in Appendix B. Institution agrees that the amounts payable or otherwise provided by GSK under this Agreement represent amounts actually and reasonably required to enable the work to be performed by Institution and Investigator in connection with the Study and have not been determined in a manner that takes into account the volume or value of any referrals or business. Institution or its authorized designee will maintain complete and accurate records of the use and disposition of the Funding. Institution represents that any additional funding it may obtain to fund the Study and/or which is used in connection with the Study will not impose any obligations, restrictions and/or conditions on Institution, Investigator and/or any Participating Site or Sub-investigator that may conflict with the rights of GSK and LIXTE (including rights of GSK and LIXTE under Section 13 below and/or the obligations of Institution and Investigator set out in this Agreement). |
(i) Institution agrees and acknowledges that the Funding received from GSK will not be used to support any type of medical education programs (e.g., Continuing Medical Education, Independent Medical Education).
| Page 3 of 38 Confidential |
(ii) No services by Institution or Investigator or any payments by GSK to Institution under this Agreement are related to or for any promotional or marketing activities.
(iii) Institution shall receive Funding from GSK on behalf of the Participating Site(s) and shall ensure that the Participating Site(s) are promptly and accurately reimbursed for their participation in this Study. GSK shall have no further responsibility to provide any further financial reimbursement to either Institution or any Participating Site(s) over and above that set out in this Agreement.
| 3.5. | Declaration of GSK and LIXTE Support. Subject to Section 11 below, Institution agrees to accurately describe GSK’s and LIXTE ‘s support for the Study in accordance with all applicable laws, regulations and institutional or publication policies applicable to the activities authorized by this Agreement. Institution agrees that: (a) all claims that Institution submits for reimbursement to any federal healthcare program or third party payor for any procedure that involves the Funding or that involves any Investigational Products provided by or on behalf of GSK and/or LIXTE at no cost to Institution will accurately reflect the provision of such Funding or supply by or on behalf of GSK and/or LIXTE; and (b) Institution will not seek reimbursement from any federal healthcare program or third party payor for any amounts paid, or Investigational Products supplied, by GSK and/or LIXTE under this Agreement. |
| 4. | Reports, Audits and Use of Study Data and Biological Samples. |
| 4.1. | Reports and Audits. Institution will maintain complete and up-to-date medical and other records relating to the Study and will keep GSK and LIXTE informed of the Study’s results and enrollment status through written reports to be provided monthly or as otherwise reasonably requested by GSK and/or LIXTE. The information to be provided in such reports will include the number of subjects enrolled in the Study and achievement of Study milestones. Subject to Section 11.1 below, Institution and/or Investigator will collect Study Data (as defined in Section 4.2 below) from the Participating Sites and will provide all Study Data (collected by Institution and Participating Sites), suitably de-identified, (as defined in HIPPA), to GSK and LIXTE (within such timeframe as is agreed in writing with GSK and LIXTE) after such Study Data is recorded in the Study database after final database freeze, or at such time that the Investigator provides the Study Data from the Study to a third party to the extent permitted hereunder, or upon termination of this Agreement as provided in Section 14.3, whichever is the earliest date. The Study Data will be provided in such format as GSK and LIXTE may reasonably request. Institution shall cause Investigator to provide each of GSK and LIXTE with a final Study report in the form of a draft manuscript, detailing the methodology, results and containing an analysis of the Study and drawing appropriate conclusions, conforming to International Committee of Medical Journal Editors guidelines and suitable for submission to a peer-reviewed journal within ninety (90) days after completion or early termination of the Study, whichever occurs first, and such manuscript will be submitted to GSK and LIXTE in accordance with Section 13 of this Agreement. At mutually agreeable times during normal administrative business hours and upon advance notice during the Study and for a period of one (1) year after completion of Study, Institution will give GSK and LIXTE and their respective designees access to all records and documentation (however stored) relating to the Study or to the care of Study subjects, in order for GSK and LIXTE to monitor the Study for source document verification and/or audit purposes. Institution and/or Investigator will also make those records and documents available for the purposes of any audit by a regulatory authority and agree not to destroy those records and documents without first giving GSK and LIXTE written notice and the opportunity to store them at GSK’s and/or LIXTE’s expense. Institution will ensure that all transactions under this Agreement are properly and accurately recorded in all material respects on its books and records and each document upon which entries in such books and records are based is complete and accurate in all material respects. Institution will maintain a system of internal accounting controls reasonably designed to ensure that it maintains no off-the-books accounts. GSK’s and/or LIXTE’s access rights under this Agreement shall be subject to GSK’s and/or LIXTE’s compliance, in connection with accessing Institution’s systems or at Institution’s facility, with Institution’s reasonable measures for purposes of confidentiality, safety, and security, and will be further subject to GSK’s and/or LIXTE’s compliance with Institution’s premises rules that are generally applicable to all persons at Institution’s facilities. Should GSK and/or LIXTE utilize one or more third party(s) in exercising its audit or inspection rights in this paragraph, GSK and/or LIXTE will ensure that such party(ies) shall be subject to an obligation of confidentiality consistent with the obligations of confidentiality required of GSK and/or LIXTE hereunder and such third party(ies) shall be subject to any and all conditions upon GSK’s and/or LIXTE’s access rights that are set forth under this Agreement. If GSK and/or LIXTE obtains, learns of, comes in contact with, or otherwise has access to any patient health and medical information in connection with the Study, GSK and/or LIXTE will keep such information confidential and will comply with all applicable laws regarding the confidentiality of such information, and GSK and/or LIXTE will not use or disclose such patient health and medical information in a manner that would violate any applicable law (including HIPAA) if such use or disclosure were made by Institution. References to “business days” in this Agreement means calendar days excluding Saturdays and Sundays and Study site holidays observed by Study site’s administrative staff, and “business hours” will refer to standard administrative work hours (generally between 8:00 am and 5:00 pm local time) on such business days. |
| Page 4 of 38 Confidential |
| 4.2. | Use of Study Data. Institution shall own the Study Data and shall ensure that the Participating Site(s) and all Study Personnel, including Investigator and Sub-investigator(s), assign all right, title and interest in Study Data to Institution (or to Participating Site(s) for further assignment to Institution). Institution hereby grants to GSK, LIXTE, and their respective affiliates a fully sublicensable, royalty-free, fully paid-up, perpetual, irrevocable, transferable, worldwide right and non-exclusive license to use all data and information resulting from the Study, including any and all data and information arising from any analyses of Biological Samples (as defined below) conducted by or on behalf of Institution, Investigator and Sub-investigator(s) in accordance with the Protocol (collectively the “Study Data”), for any and all purposes, including for inclusion in GSK’s and LIXTE’s or their respective affiliates’ patent filings and regulatory submissions for their respective Investigational Products. All other data collected or created pursuant to or prepared in connection with the Protocol other than Study Data, including medical records, laboratory notebooks, and source documents, and all other primary data sources underlying data recorded on the case report forms (CRFs) (collectively “Source Records”) shall remain at Institution as property of Institution and shall be available for inspection in accordance with Section 4.1. Institution shall have the right to publish Study Data only in accordance with Section 12 below and following any such publication Institution shall have the right to use such published Study Data for any purpose. Institution shall then have the right to use any unpublished Study Data only for internal, non-commercial research purposes and/or in connection with Study subject care. Institution discloses the Study Data to GSK and LIXTE in confidence, and GSK and LIXTE should keep the Study Data confidential until the earlier of (i) publication or other public presentation of such Study Data by Institution in accordance with Section 12 of this Agreement, or (ii) twelve (12) months after the conclusion of the Study. Notwithstanding the foregoing, GSK and LIXTE and their respective affiliates may (a) perform analysis of such Study Data as GSK and LIXTE, respectively, deems appropriate, and GSK and LIXTE, respectively, may use such Study Data and analyses as it wishes for its internal purposes, which shall include, without limitation, disclosure to any third parties to the extent that they may be assisting GSK or LIXTE or their respective affiliates with any analysis, subject to that third party agreeing to keep such Study Data and analyses confidential on the same terms as provided herein, and (b), with the consent of the other Parties, not to be unreasonably withheld, include such Study Data in patent filings and regulatory submissions for their respective Investigational Products. Institution and/or Investigator will ensure that GSK and LIXTE are named in the ICF(s) (as defined in Section 5 below) and in the HIPAA authorization form(s) or analogous documents if signed separately from the ICF (“HIPAA Authorization(s)”) (each, a “Consent Document”), as parties to whom Study subjects’ protected health information (as that term is defined in HIPAA) (“PHI”) may be disclosed in connection with the Study, and that such Consent Document(s) will permit GSK, LIXTE and their respective designees access to Study subjects’ PHI and Source Records as may be necessary to audit the Study and to use the Study Data and Biological Samples (defined in Section 4.3 below), including for research and drug development purposes. Subject to Institution’s rights herein to publish Study Data and to use such published Study Data, Institution shall not and shall procure that the Participating Site(s) shall not, give access to the unpublished Study Data to any third party other than GSK (or GSK’s nominees) or LIXTE (or LIXTE’s nominees) during the term of this Agreement and for a period of five (5) years from the date of completion of the Study without the prior written consent of GSK and LIXTE. Institution shall notify all organizations to whom it grants access to the Study Data of its obligations under this Section and shall require such organizations to give similar undertakings for the benefit of GSK and LIXTE. Notwithstanding anything to the contrary herein, GSK and LIXTE shall access, disclose, and use any patient identifiable information provided to it/them or which it/they obtains or comes in contact with under this Agreement only as (1) authorized by an applicable Study subject’s ICF and/or HIPAA authorization; and (2) as permitted by applicable law and Institution’s IRB. |
| 4.3. | Biological Samples. |
| 4.3.1. | Definition. “Biological Samples” means blood, fluid and/or tissue samples collected from Study subjects for purposes of the Study as set forth in the Protocol, and tangible materials directly or indirectly derived from such samples. |
| 4.3.2. | Use of Biological Samples. Institution will collect, retain and/or use Biological Samples only as set forth in the Protocol. Institution and/or Investigator will provide GSK and LIXTE with quantities of Biological Samples as required by the Protocol. GSK and LIXTE may use such Biological Samples as specified in the Protocol and as permitted in the Consent Documents and by applicable law. The Consent Document(s) will permit GSK, LIXTE and their respective affiliates and designees access to Biological Samples, including for research and drug development purposes. |
| 4.3.3. | Stored Biological Samples. Institution and/or Investigator are permitted, at their own expense, to transfer Biological Samples to a licensed biorepository and store the Biological Samples at the licensed biorepository after completion or early termination of the Study (the “Stored Biological Samples”). During the Term of this Agreement, Institution and Investigator may use the Stored Biological Samples as specified in the Protocol and as permitted in the Consent Documents and by applicable law. All uses of Stored Biological Samples and any data or information arising therefrom are subject to the terms of this Agreement. |
| Page 5 of 38 Confidential |
| 4.3.4. | Non-Permitted Use of Stored Biological Samples. Institution and/or Investigator will not use, and will ensure that the Participating Site(s) and other Study Personnel will not use, Biological Samples or Stored Biological Samples for any purpose other than as permitted under this Agreement and the Protocol. In the event that Institution, Participating Site(s) and/or any Study Personnel (including Sub-investigator(s)) and/or Investigator) use Biological Samples or Stored Biological Samples for any purpose other than as permitted under this Agreement or the Protocol (a “Non-Permitted Use”), Institution agrees that GSK shall be the sole and exclusive owner of any and all inventions made or invented (as such term is defined under U.S. Patent Law) in connection with the Non-Permitted Use that are solely related to GSK Confidential Information or GSK’s Investigational Product. Institution agrees that LIXTE shall be the sole and exclusive owner of any and all inventions made or invented (as such term is defined under U.S. Patent Law) in connection with the Non-Permitted Use that are solely related to LIXTE Confidential Information or LIXTE’s Investigational Product. Institution agrees that any and all inventions made or invented (as such term is defined under U.S. Patent Law) in connection with the Non-Permitted Use that are related to both GSK Confidential Information and LIXTE Confidential Information or both of GSK’s and LIXTE’s Investigational Products or that are directed to the combination therapy of the GSK Investigational Product with the LIXTE Investigational Product (“Combined Non-Permitted Use Inventions”) will be the joint property of GSK and LIXTE, and GSK and LIXTE will be the exclusive joint owners of any such Combined Non-Permitted Use Inventions. In the event of any Combined Non-Permitted Use Invention, GSK and LIXTE will negotiate in good faith an agreement that addresses each of GSK’s and LIXTE’s rights and obligations related to such Combined Non-Permitted Use Invention. Institution shall and hereby does assign any and all inventions described above in accordance with the foregoing and made as a result of a Non-Permitted Use to GSK and/or LIXTE (in accordance with the foregoing allocation of ownership) and shall execute and deliver, and shall cause its employees (including Investigator) to execute and deliver, any and all documents, including those for assignment and conveyance to memorialize such ownership of GSK and/or LIXTE in the invention and of all intellectual property rights therein consistent with Section 13 below. Notwithstanding the foregoing, any inventions made or invented by Institution personnel in connection with a Non-Permitted Use that are not owned by GSK and/or LIXTE in accordance with the foregoing shall be solely owned by Institution; provided that, Institution hereby grants to GSK, LIXTE, and their respective affiliates a non-exclusive, fully paid-up, worldwide, perpetual, irrevocable, royalty-free license to use such solely-owned Institution inventions for all purposes. For the avoidance of doubt, if any Participating Site or Sub-investigator is authorized under the Protocol, the Consent Documents and applicable law to use any Biological Samples or Stored Biological Samples, Institution shall ensure that the terms of this Section 4.3.4 are incorporated, mutatis mutandis, in the contract executed by Institution and the Participating Site in accordance with Section 7.4.1, such that GSK and LIXTE receive the same rights, assignments and licenses from Participating Site(s) as GSK and LIXTE would have received if Institution had performed the activities conducted by the Participating Site(s). |
| 5. | Institutional Review Board, Informed Consent Form, Review and Approvals. Initiation of the Study according to the Protocol shall not begin until the relevant Institutional Review Board (“IRB”) approval is obtained at Institution, and at Participating Site(s) for their Study initiation, and GSK and LIXTE have each been informed in writing of such approvals. Before submission to its IRB, Institution shall ensure Investigator supplies GSK and LIXTE with a copy of the informed consent form that is to be signed by all subjects enrolled in the Study (together with any amendments thereto, the “ICF”) for GSK’s and LIXTE ‘s review and approval. If a HIPAA Authorization form that is separate from the ICF will be used for the Study, then Institution shall ensure that Investigator also supplies GSK and LIXTE with a copy of such HIPAA Authorization form for GSK’s and LIXTE’s review and approval including, if applicable, prior to any submission to its IRB. Each Party will cooperate in the amendment of the HIPAA Authorization as may be necessary from time to time to comply with HIPAA to the extent HIPAA applies to such Party, and to ensure that the Study Data may be disclosed to and used by GSK and LIXTE and their respective affiliates and designees for the purposes contemplated by this Agreement and to the extent permitted by the IRB. Institution and/or Investigator shall inform GSK and LIXTE in writing of the IRB’s continuing reviews of the Study promptly after each such review takes place, which shall take place regularly as determined by the IRB of record and applicable law. If a Participating Site’s IRB requires material changes to its ICF and/or HIPAA Authorization that would impact the use of such site’s Study Data, Institution shall ensure that GSK and LIXTE review and approve such changes before that ICF and/or HIPAA Authorization are used at Participating Site. |
| 6. | Protocol and Informed Consent Form Changes. Institution and Investigator shall ensure that the Protocol is submitted to GSK for review and approval and submitted to LIXTE for review and approval. Institution and Investigator will not make any changes to the Protocol or the Consent Documents without first informing GSK and LIXTE of any such change and obtaining the written approval of the IRB, GSK, and LIXTE and, if necessary, making required amendments to the IND. Institution shall ensure that Investigator promptly incorporates into the ICF and Protocol any new core safety data of the Investigational Products provided by GSK and/or LIXTE and to promptly seek or procure approval of the IRB for such revised ICF. Institution, through Investigator, will be responsible for providing GSK and LIXTE with a copy of the final Protocol and Consent Documents approved by the IRB. The Protocol will be considered final after it is approved by the IRB. |
| Page 6 of 38 Confidential |
| 7. | Personnel. |
| 7.1. | Institution and Investigator. Institution represents and certifies that Institution and Investigator have the necessary experience to conduct the Study and Institution is and shall remain throughout the Study, authorized to enter into this Agreement. Investigator will lead and direct the conduct of the Study as principal investigator at Institution. |
| 7.2. | Study Personnel. Study Personnel shall include any individual by way of example, engaged by Institution, Participating Site and/or Investigator who (a) participates in the conduct of the Study; (b) is privy to information related to the scientific elements of this Study that have the potential to give rise to any Inventions (defined in Section 13.2 below) or rights related to such Inventions; and/or (c) is privy to any other Confidential Information (defined below) and such other individuals set forth in the Study proposal and Study budget provided to GSK and/or LIXTE (“Study Personnel”). For avoidance of doubt, Sub-investigator(s) (as defined in Section 7.4 below) is/are Study Personnel. |
| 7.3. | Qualified Personnel. Institution will ensure (and will cause each Participating Site (as defined below) to ensure, with respect to each Sub-investigator) that all Study Personnel conducting the Study (a) are qualified to conduct the Study; (b) are subject to the terms under this Agreement or substantially similar to those outlined under this Agreement; (c) are obligated in writing or by the terms of their employment to give ownership to Institution (or to the applicable Participating Site, in the case of each Sub-investigator) of any rights they might have in the results of their work; and (d) will do so under the direction of the Investigator at Institution and under the direction of the Sub-investigator(s) at their respective Participating Site(s), if applicable, with the prior approval and ongoing oversight of relevant competent governmental authorities. “Participating Site” means such other institution and its facilities where a Sub-Investigator is employed or under contract with, as Institution may invite to participate, or which from time to time participates, in the Study and references to “Participating Sites” shall be to any one or more of such institutions. |
| 7.4. | Sub-investigators. Each of GSK and LIXTE hereby consents to the engagement by Institution of external sub-investigator(s) (“Sub-investigator(s)”) and their Participating Sites to participate in the conduct of the Study. Institution and Investigator will provide GSK and LIXTE with a list of the Sub-investigator (s) and their Participating Site(s) promptly upon request. Institution and Investigator will notify GSK and LIXTE promptly in writing of any subsequent changes to such list and will submit IRB approval letters to GSK and LIXTE promptly for any additional Participating Site(s). In addition, Institution will: |
| 7.4.1. | contract with Participating Site(s) with respect to the Study under the terms and conditions consistent with the terms under this Agreement such that GSK and LIXTE receive the same rights from Participating Site(s) as GSK and LIXTE would have received if Institution had performed the activities conducted by the Participating Site(s), including under the terms relating to confidentiality, intellectual property (including the assignments, licenses and option rights under Section 4.3.4 and Section 13), ICFs, publication, insurance, safety reporting, GSK’s and LIXTE’s right to audit, drug accountability, Biological Samples and Stored Biological Samples and compliance with laws and regulations; |
| 7.4.2. | ensure that the Sub-investigator(s) assign to their Participating Site(s), and shall cause the Participating Site(s) to assign to Institution, any and all an obligation to assign any intellectual property made by Sub-investigator(s) as a result of their participation in the conduct of the Study in order for Institution to fulfill its obligations to GSK and LIXTE regarding inventions under Section 4.3.4 and Inventions (as defined in Section 13.2 below) under this Agreement; and |
| Page 7 of 38 Confidential |
| 7.4.3. | be solely responsible for providing shipping information to GSK and LIXTE for their distribution of Investigational Products to Sub-investigator(s), and for providing accountability information from Sub-investigator(s) for Investigational Products to the extent permitted by applicable law. |
| 8. | No Conflicts or Debarment. Institution will ensure that Institution, its trustees, officers and directors, Investigator, Participating Site(s), Sub-investigator(s), and Study Personnel: (a) are under no contractual or other obligation or restriction that is inconsistent with Institution’s and Investigator’s performance of or obligations under this Agreement; and (b) do not have a financial or other interest in GSK or LIXTE or the outcome of the Study that might interfere with their independent judgment. Institution will ensure that Institution, the Study Personnel and any and all Participating Site(s), and GSK and LIXTE, respectively, will ensure that it and any of its personnel who access Institution’s facilities, premises, or systems under this Agreement have not been, and are not under consideration to be (i) debarred from providing services pursuant to Section 306 of the United States Federal Food, Drug and Cosmetic Act 21 U.S.C. § 335a; (ii) excluded, debarred or suspended from, or otherwise ineligible to participate in any federal or state health care programs or federal procurement or non-procurement programs (as that term is defined in 42 U.S.C. § 1320a-7b(f)); (iii) disqualified by any government or regulatory agencies from performing specific services, and are not subject to a pending disqualification proceeding; or (iv) convicted of a criminal offense related to the provision of health care items or services, or under investigation or subject to any such action that is pending. During the Study and for a period of two (2) years following completion or early termination of the Study, each Party will notify the other Parties promptly if such party or any of its personnel involved in the conduct of the Study (or in the case of GSK and LIXTE any of their respective personnel who access Institution’s facilities, premises, or systems under this Agreement) are subject to the foregoing, or if any action, suit, claim, investigation, or proceeding relating to the foregoing is pending, or to the best of such Party’s knowledge, is threatened. |
| 9. | Safety Data Reporting. |
| 9.1. | Reporting. Institution is responsible for reporting all serious adverse events (SAE) (as defined in the Protocol) to regulatory authorities in the appropriate time frame as required by applicable law and as outlined in the Protocol and Appendix F. |
| 9.2. | Institution will promptly notify GSK and LIXTE of all SAEs, Special Situations (as defined in the Protocol), pregnancies, exposure via breastfeeding, and other safety information in accordance with the timelines and procedures specified in the Protocol and Appendix F. In addition, Institution will reasonably obtain and provide follow-up information as available, to GSK and LIXTE upon request. |
| 9.3. | Follow-Up Information. Institution and Investigator will assist GSK and LIXTE in investigating any SAE and will provide any follow-up information reasonably requested by GSK and LIXTE and consistent with applicable law. |
| 9.4. | Regulatory Reporting. Reporting an SAE to GSK and LIXTE does not relieve Investigator or Institution of responsibility for reporting it to the applicable regulatory authority, if and as required. |
| 10. | Communications with Regulatory Agencies. Institution shall ensure that the Investigator will |
(a) notify GSK and LIXTE of any communications from or to any regulatory authority received by the Investigator, including any safety reports, having an impact on the Investigational Products and the Study;
(b) include GSK and LIXTE in any discussions (other than immaterial discussions, such as for scheduling purposes) or meetings between Investigator and the FDA and/or other regulatory agencies regarding the Study unless prohibited by law or such regulatory agencies;
| Page 8 of 38 Confidential |
(c) supply GSK and LIXTE with a copy of any correspondence from the FDA received by the Investigator regarding the Study, including any IND, approval letter, and any other IND-related correspondence unless prohibited by law or such regulatory agencies; and inform GSK and LIXTE (and provide a copy to GSK and LIXTE, if written) of (i) any communications from a regulatory and/or government agency regarding the performance of the Study, unless prohibited by law or such regulatory agencies; (ii)any request made by a regulatory authority to audit or inspect the Study Data/Participating Site(s) and/or the activities of the Investigator, relating to the Study; and/or (iii) the withdrawal or amendment of any IRB approval/regulatory authorization relating to the Study;
(d ) allow GSK and LIXTE a reasonable opportunity to comment on any correspondence being sent by the Investigator for submission to the FDA regarding the Study, including any submitted IND and IND annual reports.
Notwithstanding anything to the contrary herein, Institution does not provide GSK or LIXTE any records maintained by Institution’s Investigational New Drug Office or any access thereto.
| 11. | Confidentiality. |
| 11.1. | Medical Confidentiality. The Parties agree to adhere to the principles of medical confidentiality in relation to Study Subjects involved in the Study. Institution shall not and shall ensure that the Investigator does not disclose to GSK or LIXTE or their affiliates PHI pertaining to Study subjects, unless required directly or indirectly for the purpose of adverse event reporting or otherwise requested by GSK and/or LIXTE in conformance with the relevant Consent Document. GSK and LIXTE shall not disclose the identity of Study subjects disclosed to it/them for this purpose to third parties without prior written consent of the Study subject, except in accordance with the requirements of all applicable laws and statutes relating to data protection. |
| 11.2. | “Confidential Information” refers to non-public information of any kind related to the Study which is disclosed by GSK or LIXTE (each, a “Discloser”) to another Party (“Recipient”) for purposes of conducting the Study, either directly or indirectly, in writing, orally or by inspection of tangible objects, including, without limitation, information and materials regarding the Discloser’s technology, products, product candidates, research and development activities, results, compound designs or structures, manufacturing or other processes or methods, know-how, inventions or other intellectual property, the existence or content of licenses, the existence, status or content of licensing or collaboration negotiations, other agreements with third parties, information regarding facilities and financial and other business information, in each case whether or not identified or marked as “confidential” (if, by the nature of the information, it would reasonably constitute proprietary or confidential information) and as such are disclosed or made available to the Recipient. Discloser’s Confidential Information may also include information obtained by Discloser from its collaborators, customers, suppliers, vendors and other third parties who have entrusted their confidential information to Discloser. During the Study and for a period of seven (7) years after the termination or expiration of this Agreement, each Recipient agrees not to (a) publish, disseminate or otherwise disclose, deliver or make available the Confidential Information of any Discloser to any third party other than competent governmental authorities, Sub-investigator(s), Participating Site(s), other Study Personnel and GSK’s affiliates or designees (in the case of GSK’s Confidential Information) and LIXTE ‘s affiliates or designees (in the case of LIXTE’s Confidential Information), and then only for the purpose of conducting the Study; or (b) make use of any Confidential Information other than in the conduct of the Study or as permitted under this Agreement. |
| 11.3. | Exceptions to Confidential Information. The obligations of nondisclosure and limited use do not apply with respect to any of the Confidential Information that: |
| 11.3.1. | is or becomes public knowledge through no breach of this Agreement by Recipient; |
| 11.3.2. | is disclosed to Recipient by a third party entitled to disclose such information without an obligation of confidentiality to Discloser; |
| Page 9 of 38 Confidential |
| 11.3.3. | is already known to Recipient prior to disclosure hereunder, or is independently developed by Recipient without use of Discloser’s Confidential Information, as shown by Recipient’s written records or competent documentation; |
| 11.3.4. | is necessary to obtain IRB approval of Study or required to be included in the written information summary provided to Study subject(s) and/or informed consent form or as necessary to be disclosed to a Study subject or potential subject in the informed consent process; |
| 11.3.5. | is released with the prior written consent of the Discloser; or |
| 11.3.6. | is required to support the medical care of a Study subject. |
| 11.4. | Recipient may disclose the Discloser’s Confidential Information to the extent that it is required to be produced pursuant to a requirement of applicable law, IRB, government agency, an order of a court of competent jurisdiction, or a facially valid administrative, Congressional, or other subpoena, provided that subject to the requirement, order, or subpoena the Discloser is notified if legally permissible and Recipient cooperates with Discloser’s efforts to limit the scope of the information to be provided or to obtain an order protecting the information from public disclosure. To the extent allowed under applicable law, Discloser may seek to limit the scope of such disclosure and/or seek to obtain a protective order. Recipient will disclose only the minimum amount of Confidential Information necessary to comply with such requirement, law, subpoena or court order as advised by Recipient’s legal counsel. |
| 11.5. | Upon Discloser’s written request, Recipient agrees to return all Confidential Information supplied to it by Discloser, at Discloser’s expense, pursuant to this Agreement except that Recipient may retain an archival copy of such Confidential Information in a secure location for purposes of identifying and satisfying its obligations and exercising its rights under this Agreement. |
| 11.6. | Any Party may disclose the existence and terms of this Agreement as and to the extent necessary to ensure compliance with applicable Federal, State and Institutional policies, regulations, and laws, and GSK and LIXTE may disclose the existence and terms of this Agreement to their respective actual or prospective licensees, sublicensees, collaborators, strategic partners and/or acquirers under confidentiality terms at least as stringent as those hereunder (but of commercially reasonable duration). |
| 11.7. | Institution agrees to not provide GSK’s Confidential Information to LIXTE and to not provide LIXTE’s Confidential Information to GSK without GSK’s and LIXTE’s respective written consent. Notwithstanding the foregoing, to the extent either GSK or LIXTE receives Confidential Information of the other in connection with this Agreement, the obligations of non-use and non-disclosure under this Section 11 will apply to it with respect to such Confidential Information of the other Party. |
| 12. | Publication. |
| 12.1. | GSK and LIXTE recognise that Institution and the Investigator intend that results of scientific interest arising from the Study will be appropriately published and disseminated. Institution and/or Investigator may publish or disclose the results of the Study; provided, that (a) a copy of any proposed disclosure, including any publication, abstract, manuscript, note, slides, oral presentation, poster, or any other document or material whether in written, electronic or printed form and, where publication is intended to be verbal presentation only, any other copy of the intended script, oral presentation, or poster that reports all or parts (interim or final) of the results of the Study or Study progress, is given to GSK and LIXTE for review at least thirty (30) days prior to the date of submission for publication (including abstracts) or of public disclosure; (b) if requested by GSK in writing during such review period, any reference to GSK’s Confidential Information is deleted, and if requested by LIXTE in writing during such review period, any reference to LIXTE’s Confidential Information is deleted; and (c) Institution and/or Investigator defer publication or disclosure for up to an additional sixty (60) days from the time GSK and/or LIXTE notifies Institution and/or Investigator in writing during such review period that GSK and/or LIXTE (i) desires patent application(s) to be filed on any Invention described in the proposed disclosure or (ii) desires to take any other measures as GSK or LIXTE considers necessary to establish, register, protect and/or preserve any intellectual property relating to the GSK Investigational Product and/or LIXTE Investigational Product. Institution and Investigator will ensure that any publication or public disclosure of the results of the Study proposed by a Sub-investigator(s) is/are made in accordance with this Section 12. |
| Page 10 of 38 Confidential |
| 12.2. | Institution and/or Investigator agrees that all reasonable comments made by GSK or LIXTE in relation to a proposed publication by Institution and/or Investigator will be reasonably considered for incorporation by Institution and/or Investigator into the publication. Institution and/or Investigator shall ensure that any of GSK’s Confidential Information is removed from the proposed publication and any of LIXTE’s Confidential Information is removed from the proposed publication. |
| 13. | Intellectual Property. |
| 13.1. | Pre-Existing Intellectual Property. It is expressly agreed that no Party transfers to another Party by operation of this Agreement any patent right, copyright, or other proprietary or property right of any kind any Party owns as of the Effective Date. Nothing herein is intended to grant to any Party any license or other rights in and to such pre-existing or independently developed intellectual property of another Party, except for Institution’s limited right to use the Investigational Products, LIXTE Confidential Information, and GSK Confidential Information solely for the purposes of conducting the Study. |
| 13.2. | Inventions/Investigational Product Inventions. “Inventions” means any inventions, discoveries, know-how, and improvements (including new uses and improvements of the Investigational Products), and all intellectual property rights therein, whether or not protectable under patent or other intellectual property law, that are made or invented by Institution personnel, Investigator, Study Personnel (alone or with others), or any Participating Site(s) personnel, or Sub-investigator(s), (a) during and resulting from performance of the Study or the use of the GSK Investigational Product and/or the LIXTE Investigational Product; or (b) through the use of GSK’s Confidential Information and/or LIXTE’s Confidential Information under this Agreement during the Term of this Agreement and during the secrecy period described in Section 11.2 above. Each Party agrees that: |
13.2.1 any Invention that incorporates, is based on, refers to or relates solely to GSK Confidential Information or solely to the GSK Investigational Product, or the modification, use or manufacture thereof, (including Inventions that generically encompass within their scope the GSK Investigational Product, or Inventions relating to the use of the GSK Investigational Product), including any Invention that involves identification or use of biomarkers related to the safety, efficacy, or use of the GSK Investigational Product, but excluding Combined Therapy Inventions (as defined in Section 13.2.3 below) (“GSK Investigational Product Inventions”) shall be promptly disclosed by Institution to GSK in writing (and Institution shall ensure that any GSK Investigational Product Invention is promptly disclosed to Institution by all Study Personnel and Participating Site(s)) and shall be the sole and exclusive property of GSK. Institution shall assign and does hereby assign to GSK all right, title, and interest in the United States and throughout the world to all GSK Investigational Product Inventions (and, for the avoidance of doubt, Institution shall ensure that all Study Personnel, including Investigator and Sub-investigator(s), have assigned to Institution or Participating Site(s), as applicable, (and that Participating Site(s) have assigned to Institution) all right, title, and interest in the United States and throughout the world to all GSK Investigational Product Inventions). As agreed solely between GSK and LIXTE and without any obligation on the part of Institution, LIXTE will have no right and license to Study Data generated from the use of and related solely to GSK’s Investigational Product (for clarity, not in combination with LIXTE’s Investigational Product) or GSK Confidential Information, without GSK’s written consent.
| Page 11 of 38 Confidential |
13.2.2 any Invention that incorporates, is based on, refers to or relates solely to LIXTE Confidential Information or solely to the LIXTE Investigational Product, or the modification, use or manufacture thereof, (including Inventions that generically encompass within their scope the LIXTE Investigational Product, or Inventions relating to the use of the LIXTE Investigational Product), including any Invention that involves identification or use of biomarkers related to the safety, efficacy, or use of the LIXTE Investigational Product, but excluding Combined Therapy Inventions (“LIXTE Investigational Product Inventions”) shall be promptly disclosed by Institution to LIXTE in writing (and Institution shall ensure that any LIXTE Investigational Product Invention is promptly disclosed to Institution by all Study Personnel and Participating Site(s)) and shall be the sole and exclusive property of LIXTE. Institution shall assign and does hereby assign to LIXTE all right, title, and interest in the United States and throughout the world to all LIXTE Investigational Product Inventions (and, for the avoidance of doubt, Institution shall ensure that all Study Personnel, including Investigator and Sub-investigator(s), have assigned to Institution or Participating Site(s), as applicable, (and that Participating Site(s) have assigned to Institution) all right, title, and interest in the United States and throughout the world to all LIXTE Investigational Product Inventions). As agreed solely between GSK and LIXTE and without any obligation on the part of Institution, GSK will have no right and license to Study Data generated from the use of and related solely to LIXTE’s Investigational Product (for clarity, not in combination with GSK’s Investigational Product) or LIXTE’s Confidential Information without LIXTE’s written consent.
13.2.3 any Invention that relates to both GSK Confidential Information and LIXTE Confidential Information or to both the GSK Investigational Product (or the modification, use or manufacture thereof) and the LIXTE Investigational Product (or the modification, use or manufacture thereof) as a combination therapy (“Combined Therapy Inventions”) will:
(i) be promptly disclosed by Institution to both GSK and LIXTE in writing (and Institution shall ensure that any Combined Therapy Invention is promptly disclosed to Institution by all Study Personnel and Participating Site(s)); and
(ii) shall be the joint property of GSK and LIXTE, and GSK and LIXTE shall be the exclusive joint owners of Combined Therapy Inventions. Institution shall assign and does hereby assign to GSK and LIXTE jointly all right, title, and interest in the United States and throughout the world to all Combined Therapy Inventions (and, for the avoidance of doubt, Institution shall ensure that all Study Personnel, including Investigator and Sub-investigator(s), have assigned to Institution or Participating Site(s), as applicable, (and that Participating Site(s) have assigned to Institution) all right, title, and interest in the United States and throughout the world to all Combined Therapy Inventions). In the event of any Combined Therapy Invention, GSK and LIXTE will negotiate in good faith an agreement that addresses each of GSK’s and LIXTE’s rights and obligations related to such Combined Therapy Invention.
| 13.3. | Institution will, and will cause Study Personnel (including Investigator), and will ensure that all Participating Site(s) will, and will cause the relevant Sub-investigator(s), to: |
(a) reasonably cooperate in the preparation of applications or registrations for patent rights and other proprietary protection for any and all patentable or protectable GSK Investigational Product Inventions, LIXTE Investigational Product Inventions, and Combined Therapy Inventions all in the name of (i) GSK for GSK Investigational Product Inventions, (ii) LIXTE for LIXTE Investigational Product Inventions, and (iii) GSK and LIXTE jointly for Combined Therapy Inventions, at
(x) GSK’s cost and expense for GSK Investigational Product Inventions;
(y) LIXTE’ cost and expense for LIXTE Investigational Product Inventions; and
(z) GSK’s and LIXTE’s cost and expense on a 50%/50% basis for Combined Therapy Inventions; and
(b) execute and deliver all requested applications, assignments, and other documents and take such other measures as GSK and/or LIXTE reasonably requests, that are necessary in order to perfect GSK’s and LIXTE’s respective rights in GSK Investigational Product Inventions, LIXTE Investigational Product Inventions, and Combined Therapy Inventions.
| Page 12 of 38 Confidential |
| 13.4. | Other Inventions. Inventions (as defined in Section 13.2) that are not GSK Investigational Product Inventions, LIXTE Investigational Product Inventions or Combined Therapy Inventions (the “Other Inventions”) shall be owned in accordance with inventorship as determined under U.S. Patent Law. |
| 13.5. | License and Options. In the event Institution has an ownership interest in any Other Invention, Institution, on behalf of itself, all Participating Site(s), Investigator and all other Study Personnel (including each Sub-investigator(s)), hereby grants to each of GSK and LIXTE and their respective affiliates a non-exclusive, fully paid-up, worldwide, perpetual, royalty-free, irrevocable license to use Other Inventions for all purposes. In addition, Institution, on behalf of itself, all Participating Site(s), Investigator and all other Study Personnel (including each Sub-investigator(s)), hereby grants to each of GSK and LIXTE a first option to obtain a co-exclusive (exclusive to GSK and LIXTE) perpetual, irrevocable, transferable, royalty-bearing and sublicensable (through multiple tiers) license (“Co-Exclusive License”) to Institution’s, Investigator’s, Study Personnel’s or any Participating Site’s or Sub-investigator’s, interest in any Other Inventions to make, use and sell (and otherwise research, develop and commercialize) those inventions or any products that are covered by patent rights that claim or include those inventions. GSK’s and LIXTE’s Co-Exclusive License option may be exercised with respect to an Other Invention by notice in writing from GSK and/or LIXTE to each of the other Parties, at any time during a period of one-hundred & twenty (120) days (the “Option Period”) after the full written disclosure to GSK and LIXTE by Institution of each such Other Invention. Upon GSK and LIXTE both exercising their Co-Exclusive License with regard to any particular Other Invention, Institution, LIXTE and GSK will negotiate in good faith in an attempt to reach a license agreement satisfactory to all Parties (the “Negotiation Period”). Unless extended by the written consent of all of the Parties, the Option Period and the Negotiation Period shall not exceed two-hundred & forty (240) days in the aggregate. If either GSK or LIXTE declines to exercise the Co-Exclusive License option during an Option Period, then GSK or LIXTE, as the case may be, may exercise the Co-Exclusive License option alone which may result in an exclusive license to such Party. In the event that an Option Period lapses or, including any extensions, terminates, Institution may enter into negotiations to enter into a non-exclusive license agreement with a third party without further obligation to GSK or LIXTE under this Agreement with regard to Institution’s interest in such Other Inventions; provided, however, that the terms offered to a third party are not more favorable than last offered to GSK or LIXTE and provided further that the non-exclusive licenses to GSK and LIXTE will remain in full force and effect. |
| 13.6. | Notwithstanding anything to the contrary, Institution will retain a non-exclusive, royalty-free license to internally use any Invention to which Institution’s personnel or any Participating Site’s personnel makes an inventive contribution for Institution’s research, academic and patient care purposes, which Institution can sublicense only to the contributing Participating Site for such Participating Site’s research, academic and patient care purposes. |
| 14. | Term and Termination; Completion. |
| 14.1 | Term. This Agreement is effective as of the Effective Date and will continue in effect through completion of the Study, unless earlier terminated pursuant to this Section 14 (“Term”). |
| 14.2 | Termination. This Agreement may be terminated by any Party without cause upon thirty (30) days prior written notice to the other Parties. This Agreement may be terminated by any Party (a) immediately upon written notice to the other Parties if necessary to protect the safety, health or welfare of subjects enrolled in the Study or upon withdrawal of regulatory authorization for the Study; or (b) for a breach of a material provision hereof by a Party, which breach is not cured within thirty (30) days following receipt of written notice thereof; or (c) immediately upon written notice to the other Parties if Investigator is no longer able (for whatever reason) to act as Investigator and no replacement mutually acceptable to Institution, GSK and LIXTE can be found; or (d) if a Party becomes insolvent, or if an order is made or a resolution is passed for its winding up (except voluntarily for the purpose of solvent amalgamation or reconstruction), or if an administrator, administrative receiver or receiver is appointed over the whole or any part of its assets, or if it makes any arrangement with its creditors. Additionally, GSK or LIXTE shall be entitled to terminate this Agreement immediately on written notice to Institution and the other Party if Institution fails to perform its obligations in accordance with Section 17 below. Except for payment due to the Institution as detailed in this Agreement, including but not limited to Section 14.3 below and further set out in Appendix B, Institution shall have no claim against GSK or LIXTE for compensation for any loss of whatever nature by virtue of the termination of this Agreement in accordance with this Section 14.2. |
| Page 13 of 38 Confidential |
| 14.3 | Effect of Termination/Completion of Study. Upon either an early termination under Section 14.2 or completion of the Study, (a) this Agreement will terminate; (b) Investigator will immediately stop enrolling subjects into the Study and determine the appropriate manner to cease conducting Study procedures and administration of the Investigational Products to subjects already entered into the Study; (c) Institution will provide GSK with a reconciliation of Study costs against budget as set forth in Appendix B; (d) Institution and/or Investigator will provide a copy of all Study Data to GSK and LIXTE and will return to GSK all of GSK’s Confidential Information and return to LIXTE all of LIXTE’s Confidential Information, except that Institution may retain one archival copy of each of GSK Confidential Information and LIXTE Confidential Information solely for purposes of determining future compliance with the terms of this Agreement and as necessary for legal, regulatory, compliance, or insurance purposes; (e) to the extent GSK and/or LIXTE received Confidential Information of another Party in connection with this Agreement, each of GSK and/or LIXTE, as applicable, will return the Confidential Information to the owning Party; and (f) GSK shall pay Institution all such amounts incurred or obligated by Institution in accordance with milestones as set out in Appendix B at the effective date of such termination. |
| 14.4 | Survival. No expiration or termination of this Agreement will release the Parties from their rights and obligations accrued prior to the effective date of expiration or termination. The rights and duties under Sections 3.1(f) (Investigational Products Supply and Accountability); 3.5 (Declaration of GSK and LIXTE Support); 4 (Reports, Audits and Use of Study Data and Biological Samples); 8 (No Conflicts or Debarment) (for the period of time set forth therein); 9 (Safety Data Reporting); 10 (Communications with Regulatory Agencies); 11 (Confidentiality) (for the period of time set forth therein), 12 (Publication), 13 (Intellectual Property); 14.3 (Effects of Termination); 14.4 (Survival); 15 (Indemnification, Remedies, Insurance and Study Subject Injury); 17.2 (Antibribery) and 18 (Miscellaneous) will survive the termination of this Agreement. |
| 15. | Indemnification, Remedies, Insurance and Study Subject Injury. |
| 15.1. | Indemnification by GSK. GSK will indemnify, defend and hold harmless Institution, System, and their Regents, directors, officers, employees (including Investigator), agents, Participating Site(s) and Sub-investigator(s), and any other third parties engaged at their discretion, (collectively, the “Institution Indemnitees”) and LIXTE, its directors, officers, employees and agents (collectively, the “LIXTE Indemnitees”) against any third party claims, including losses, liabilities, damages, claims, costs, charges, expenses and reasonable attorney’s fees for defending those claims (each, a “Claim”) to the extent a Claim arises out of or relates to (i) the personal injury (including death) or property damage arising out of or connected with GSK’s failure to manufacture and provide the GSK Investigational Product in accordance with current Good Manufacturing Practices (“GMP”), (ii) the use by GSK of the results of the Study, and (iii) the activities to be carried out by GSK under this Agreement. GSK’s obligations under this Section 15.1 will not apply to the extent that a Claim is indemnifiable by LIXTE or Institution under Section 15.2 or Section 15.3, respectively, or arises out of or relates to: (a) an Institution Indemnitee’s or a LIXTE Indemnitee’s respective (i) negligence, gross negligence or willful misconduct, or (ii) failure to adhere to the terms of this Agreement or any reasonable written instructions from GSK or its designee; or (b) an Institution Indemnitee’s (1) failure to conduct the Study in accordance with applicable laws or regulations, or (2) failure to adhere to the terms of the Protocol or any reasonable written instructions from GSK or its designees; or (c) any materials, equipment, device and/or drug products used in the Study that are not manufactured or provided by GSK. |
| Page 14 of 38 Confidential |
| 15.2. | Indemnification by LIXTE. LIXTE will indemnify, defend, and hold harmless and the Institution Indemnitees and GSK, its directors, officers, employees and agents (collectively, the “GSK Indemnitees”) against any Claim to the extent such Claim arises out of or relates to (i) the personal injury (including death) or property damage arising out of or connected with LIXTE’s failure to manufacture and provide the LIXTE Investigational Product in accordance with GMP, (ii) the use by LIXTE of the results of the Study, and (iii) the activities to be carried out by LIXTE under this Agreement. LIXTE’s obligations under this Section 15.2 will not apply to the extent that a Claim is indemnifiable by GSK or Institution under Section 15.1 or Section 15.3, respectively, or arises out of or relates to: (a) an Institution Indemnitee’s or a GSK Indemnitee’s respective (i) negligence, gross negligence or willful misconduct, or (ii) failure to adhere to the terms of this Agreement or any reasonable written instructions from LIXTE or its designee; or (b) an Institution Indemnitee’s (1) failure to conduct the Study in accordance with applicable laws or regulations; or (2) failure to adhere to the terms of the Protocol or any reasonable written instructions from LIXTE or its designees; or (c) any materials, equipment, device and/or drug products used in the Study that are not manufactured or provided by LIXTE. |
| 15.3. | Indemnification by Institution. To the extent authorized under the Constitution and the laws of the State of Texas, Institution will indemnify, defend, and hold harmless the GSK Indemnitees and the LIXTE Indemnitees against any Claim to the extent such Claim arises out of or relates to (a) the performance of the Study by Institution or any Institution Indemnitees; or (b) any Institution Indemnitee’s: (i) negligence, gross negligence or willful misconduct, (ii) failure to adhere to the terms of the Protocol, this Agreement, or any reasonable written instructions from GSK or LIXTE or their respective designees, or (iii) failure to conduct the Study in accordance with applicable laws or regulations; provided, however, that deviations from the Protocol for health and safety reasons shall not constitute: failure to follow to the terms of the Protocol, negligence or willful misconduct by an Institution Indemnitee or a breach of this Agreement by an Institution Indemnitee. Institution’s obligations under this Section 15.3 will not apply to the extent that a Claim is indemnifiable by GSK or LIXTE under Section 15.1 or Section 15.2, respectively, or arises out of or relates to the negligence, gross negligence, or willful misconduct of GSK Indemnitees, LIXTE Indemnitees, or any person other than an Institution Indemnitee. |
| 15.4. | Indemnification Procedure. A Party (the “indemnified Party”) must use reasonable efforts to notify the indemnifying Party within thirty (30) days of receipt of any Claim for which such indemnifying Party might be liable under Section 15.1, 15.2, or 15.3, as the case may be; provided, however, the failure to promptly notify the indemnifying Party shall not relieve the indemnifying Party of its indemnification obligations unless the indemnifying Party is materially adversely affected by such failure. The indemnifying Party will have the sole right to defend, negotiate, and settle such Claim; provided, however, such settlements shall not require the indemnified Party to contribute to the settlement, admit fault or require the indemnified Party to change its operations or business practices, without the indemnified Party’s written consent. The indemnified Party will be entitled to participate in the defense of such matter and to employ counsel at its expense to assist in such defense; provided, however, that the indemnifying Party will have final decision-making authority regarding all aspects of the defense of the Claim. The indemnified Party seeking indemnification will provide the indemnifying Party with such information and assistance as the indemnifying Party may reasonably request, at the expense of the indemnifying Party. No indemnified Party will be responsible or bound by any settlement of any Claim or suit made without its prior written consent; provided, however, that the indemnified Party will not unreasonably withhold such consent. This Section 15 is subject to the statutory duties of the Texas Attorney General. |
| Page 15 of 38 Confidential |
| 15.5. | Remedies. Institution agrees that (a) GSK and LIXTE may be irreparably injured by an impending or existing breach of this Agreement; (b) money damages may not be an adequate remedy for any such breach; and (c) GSK and LIXTE will be entitled to seek equitable relief, including injunctive relief and specific performance, without having to post a bond, as a remedy for any such breach. The provisions of this Section 15.5 are not intended to be exclusive and are without prejudice to the rights of GSK and/or LIXTE to seek any other right or remedy that it may have under this Agreement or otherwise. |
| 15.6. | Insurance. Each member of The University of Texas System is self-insured pursuant to The University of Texas Professional Medical Liability Benefit Plan under the authority of Chapter 59, Texas Education Code. Institution has and will maintain in force during the Term of this Agreement adequate insurance or financial resources to cover its indemnification obligations. Upon written request, Institution will provide written evidence of self-insurance or financial resources. |
| 15.7. | Study-Related Injury. If a Study subject is injured or becomes ill as a result of participating in the Study, Institution shall ensure that the Study subject has access to medical treatment necessary to treat such injury or illness. This Agreement does not obligate any of the Parties to provide medical treatment, except to the extent required by applicable law, nor does this Agreement obligate any Party to provide reimbursement for medical treatment if a Study subject requires medical treatment for physical illness or injury sustained as a direct result of the treatment of such Study subject in accordance with this Agreement and the Protocol. |
| 15.8. | Mitigation. Nothing in this Agreement shall relieve any party from its duty under applicable law to mitigate any loss or damage incurred by it as a result of any matter giving rise to a Claim. |
| 16. | Security Breaches and Data Privacy |
| 16.1 | Notification of Data Security Breaches. The Parties agree to notify each other in accordance with the below, without undue delay after discovery of a Security Breach (as defined below) and awareness that such Security Breach relates to this Agreement. |
| (i) | Notice of a Security Breach to GSK, will be sent via e-mail to csir@gsk.com. |
Notice of a Security Breach to LIXTE, will be sent via e-mail to: info@lixte.com
Notice of a Security Breach to Institution will be sent to: PrivacyCompliance@mdanderson.org
with a follow-up to: The University of Texas M. D. Anderson Cancer Center, Institutional Compliance Office, Unit 1640, P.O. Box 301407, Houston, Texas 77230-1407, Attn: Privacy Officer, Fax No. 713-563-4324.
| (ii) | In the course of notification to each other, the Parties will provide, as feasible, sufficient information for the Parties to jointly assess the Security Breach and, to the extent a Party or the Parties may be subject to an applicable legal requirement regarding disclosure to a competent governmental authority, make any required notification to any government authority within the timeline required by applicable laws. Such information may include, but is not necessarily limited to: |
| (a) | The nature of the Security Breach, the categories and approximate number of data subjects and records; |
| (b) | The likely consequences of the Security Breach, in so far as consequences are able to be determined; |
| (c) | Any measures taken to address or mitigate the incident. |
| (iii) | Following a breach of PHI, the Party (or Parties) who bear the regulatory duty to determine whether a Security Breach occurred will decide on the basis of all available information and applicable laws if the Security Breach will be considered a reportable Security Breach and arrange for notification to data subjects and/or government authorities, if required by applicable laws. Where the Parties decide that notification is required by applicable laws, the Party responsible for providing such notification under applicable data privacy laws shall be responsible for providing such notification. |
| Page 16 of 38 Confidential |
| (iv) | Assistance in event of Security Breach. In the event of a Security Breach relating to the personal information of another Party’s personnel and/or GSK’s or LIXTE’s Confidential Information collected or received by a Party under this Agreement, each Party agrees to assist and fully cooperate with the other Party with respect to any internal or external investigation through the provision of access to information, employees, interviews, materials, databases, or any and all other items required to fully investigate and resolve any such incidents and provide information necessary to enable required notifications. The breached Party agrees to take such remedial actions as the Parties mutually agree is warranted. Notwithstanding the foregoing, the Parties will each comply with HIPAA, to the extent applicable, with regards to any breach that involves PHI. |
| (v) | No Party shall disclose, without the other Parties’ prior written approval, any information related to the suspected Security Breach to any third party other than a vendor hired to investigate/mitigate such Security Breach and bound by confidentiality obligations, except as required by applicable laws. |
| (vi) | “Security Breach” means any actual or suspected breach of security leading to the accidental or unlawful destruction, disclosure of, or access to, the Confidential Information or personal data of another Party’s personnel, as applicable, of any Party. |
| 17. | Anti-bribery and Corruption |
| 17.1 | Institution shall comply, and shall ensure Investigator complies, fully at all times with all applicable laws and regulations, including but not limited to anti-corruption laws, and that it has not, and covenants that it will not, in connection with the performance of this Agreement, directly or indirectly, make, promise, authorize, ratify or offer to make, or take any act in furtherance of any payment or transfer of anything of value for the purpose of influencing, inducing or rewarding any act, omission or decision to secure an improper advantage; or improperly assisting it or GSK or LIXTE in obtaining or retaining business, or in any way with the purpose or effect of public or commercial bribery, and warrants that it has taken reasonable measures to prevent subcontractors, agents or any other third parties, including Participating Site(s) and Sub-investigator(s), subject to its control or determining influence, from doing so. For the avoidance of doubt this includes facilitating payments, which are unofficial, improper, small payments or gifts offered or made to government officials to secure or expedite a routine or necessary action to which we are legally entitled. |
| 17.2 | Institution agrees that in the event that GSK or LIXTE believes that there has been a possible violation of the terms of this Section 17, GSK or LIXTE may make full disclosure of such belief and related information at any time and for any reason to any competent government bodies and their agencies, and, subject to the confidentiality obligations herein and with respect to any PHI, to the extent permitted by any applicable Consent Document and applicable law, to whomever GSK or LIXTE determines in good faith has a legitimate need to know. |
| 18. | Miscellaneous. |
| 18.1 | Independent Contractor. Institution, including Investigator, is an independent contractor and, as such, none of Institution, Institution’s employees, or Investigator will be entitled to any benefits applicable to employees of GSK and/or LIXTE. No Party is authorized or empowered to act as agent for any other Party for any purpose under this Agreement and will not, on behalf of another Party, enter into any contract, warranty or representation as to any matter herein. No Party will be bound by the acts or conduct of any other Party. Nothing in this Agreement and no action taken by the Parties under this Agreement shall constitute a partnership, association or other co-operative entity between any of the Parties or make any Party the agent of any other Party for any purpose. |
| Page 17 of 38 Confidential |
| 18.2 | Use of Names; Publicity. Except to the extent required by applicable law or regulation or the rules of any stock exchange or listing agency, no Party will use the name of another Party in any form of advertising, promotion, social media or publicity or in any press release, without the prior written consent of that other Party. Institution agrees and will ensure that Study Personnel agree not to answer third party inquiries regarding the Study or the Investigational Products from financial analysts other than pursuant to a requirement of applicable law, IRB, government agency, an order of a court of competent jurisdiction, or a facially valid administrative, Congressional, or other subpoena and only in accordance with Section 11.4. |
| 18.3 | Sponsorship. Institution agrees that GSK or LIXTE will not be listed as the sponsor of the Study in any documentation related to the Study and will ensure that GSK or LIXTE is not identified as the sponsor for regulatory purposes nor referenced to have assumed any sponsor responsibilities for this Study. |
| 18.4 | Certain Disclosures and Transparency. Institution acknowledges that GSK and its affiliates and LIXTE and its affiliates are required to abide by applicable federal and state disclosure laws and GSK transparency policies governing their activities including providing reports to the government and to the public concerning financial or other relationships with healthcare providers and healthcare organizations. Institution agrees that GSK and its affiliates and LIXTE and its affiliates may, in their sole discretion, disclose information about this Agreement and about the Study as required by law, including relating to any transfers of value pursuant to this Agreement. Institution agrees to supply information reasonably requested by GSK and LIXTE for disclosure purposes, other than information that Institution previously provided to GSK. To the extent that Institution is independently obligated to disclose specific information concerning the Study, including relating to transfers of value from GSK or its affiliates pursuant to this Agreement, Institution will make timely and accurate required disclosures. Institution hereby represents that Investigator is aware of the possibility of such disclosure. |
(i) GSKs disclosure link is as follows: “https://www.gsk.com/en-gb/responsibility/ethical-standards/engaging-with-healthcare-professionals/#Disclosures”
| 18.5 | Assignment; Subcontracting. Except as expressly provided in this Agreement, Institution may not assign, delegate or transfer its obligations under this Agreement, in whole or in part, without the prior written consent of GSK and LIXTE, and any attempted assignment, delegation or transfer by Institution without such consent will be void. GSK and LIXTE may each, respectively, assign, delegate or transfer this Agreement to an affiliate or in connection with the acquisition, licensing, sale or purchase of any or all of its interests or its Investigational Product or its business or assets to which this Agreement relates, in whole or in part, without the consent of the other Parties; however, each of GSK and LIXTE will, as the case may be, provide notice to the other Parties if it assigns this Agreement. No assignment, delegation or transfer will relieve any Party of the performance of any accrued obligation that such Party may then have under this Agreement. With GSK’s and LIXTE’s prior written consent in each instance, Institution may subcontract the performance of certain of its activities under this Agreement to qualified third parties other than Sub-investigator(s) and Participating Site(s); provided, that (a) such permitted third parties perform such activities in a manner consistent with the terms and conditions in this Agreement; (b) Institution causes such permitted third parties to be bound by and comply with the terms of this Agreement, as applicable, including the obligations described in Section 7.4 above; (c) Institution remains liable for Institution’s obligations under this Agreement regardless of any delegations to such permitted third parties; and (d) other than as disclosed to GSK and LIXTE in writing, neither Investigator nor any Sub-investigator has any direct or indirect financial interest in any such permitted third parties. For the avoidance of doubt, and notwithstanding anything to the contrary herein, all permitted third parties used to perform the Study will be considered Study Personnel under this Agreement. |
| Page 18 of 38 Confidential |
| 18.6 | Notice. All notices must be in writing and sent to the address for the recipient set forth below or at such other address as the recipient may specify in writing under this procedure. All notices must be given (a) by personal delivery, with receipt acknowledged; or (b) prepaid certified or registered mail, return receipt requested; or (c) by prepaid recognized express delivery service. Notices will be effective upon receipt or at a later date stated in the notice. |
| If to GSK: | GSK LLC | |
| 1000 Winter Street, Suite 3300, Waltham, MA 02451 | ||
| Attn: Tyler Lockard | ||
| Email: external.research@gsk.com; Fax: +1.339.309.5112 | ||
| With a copy to: | ||
| GSK LLC, Marc Harris, Contracting | ||
| 1250 South Collegeville Road, Bldg 4, 4th FL | ||
| Collegeville, PA., 19426 (marc.2.harris@gsk.com) | ||
| If to LIXTE: | LIXTE Biotechnology Holdings, Inc | |
| Attn: Eric J. Forman (eforman@lixte.com) | ||
| 680 E Colorado Blvd., Suite 180 | ||
| Pasadena, CA 91101 | ||
| With a copy to: | ||
| Cooley LLP | ||
| Attn: Matthew E. Langer (mlanger@cooley.com) | ||
| 55 Hudson Yards | ||
| New York, NY 10001-2157 | ||
| If to Institution: | ||
| The University of Texas M.D. Anderson Cancer Center | ||
| 7007 Bertner Avenue, 1MC11.3343 | ||
| Legal Services, Unit 1674 | ||
| Attn: Chief Legal Officer | ||
| Houston, TX 77030 | ||
| Phone: (713) 745-6633; Facsimile: (713) 745-6029 | ||
| With a copy to: | ||
| The University of Texas M.D. Anderson Cancer Center | ||
| 7007 Bertner Ave. | ||
| Office of Sponsored Programs, Unit 1676 | ||
| Attn: Associate VP, Research Administration | ||
| Houston, TX 77030 | ||
| Phone: (713) 792-3220; Facsimile: (713) 794-4595 | ||
| Investigator: | ||
| Vice Chair for Clinical Research | ||
| Director of the Gynecologic Cancer Immunotherapy Program | ||
| Professor, Department of Gynecologic Oncology and Reproductive Medicine | ||
| The University of Texas MD Anderson Cancer Center. | ||
| 1515 Holcombe Blvd., Unit 1362 | ||
| Houston, TX 77030 | ||
| Attn: Dr. Amir Jazaeri (aajazaeri@mdanderson.org) |
| Page 19 of 38 Confidential |
| 18.7 | Entire Agreement; No Modification. This Agreement, including all Appendices which are incorporated into this Agreement, constitutes the entire agreement among the Parties with respect to the Study that is the specific subject matter of this Agreement and supersedes all prior agreements, oral or written, with respect to such subject matter. This Agreement may not be amended or modified except in a written instrument signed by an authorized representative of each of Institution, GSK and LIXTE, and acknowledged by Investigator. Any conflicts between any Appendices and this Agreement will be governed and controlled by provisions of the main text of this Agreement. |
| 18.8 | Severability; Reformation. Each provision in this Agreement is independent and severable from the others, and no provision will be rendered unenforceable because any other provision is found by a proper authority to be invalid or unenforceable in whole or in part. If any provision of this Agreement is found by such an authority to be invalid or unenforceable in whole or in part, such provision will be changed and interpreted so as to best accomplish the objectives of such unenforceable or invalid provision and the intent of the Parties, within the limits of applicable law. |
| 18.9 | Governing Law. Institution is an agency of the State of Texas and under the Constitution and the laws of the State of Texas possesses certain rights and privileges, is subject to certain limitations and restrictions, and only has such authority as is granted to it under the Constitution and laws of the State of Texas. This Agreement and any disputes arising out of or relating to this Agreement will be governed by, construed and interpreted in accordance with the Constitution and laws of the State of Texas, without regard to any choice of law principle that would require the application of the law of another jurisdiction. |
| 18.10 | Waivers. Any delay in enforcing a Party’s rights under this Agreement, or any waiver as to a particular default or other matter, will not constitute a waiver of such Party’s rights to the future enforcement of its rights under this Agreement, except with respect to an express written waiver relating to a particular matter for a particular period of time signed by an authorized representative of the waiving Party, as applicable. To clarify, any such waiver by GSK, LIXTE, or Institution must be evidenced by an instrument in writing executed by an officer of such party authorized to execute waivers. |
| 18.11 | Rights Cumulative. The rights and remedies contained in this Agreement are cumulative and not exclusive of any rights or remedies provided by law. |
| 18.12 | Party Rights. Except as specifically provided in this Agreement, nothing expressed or implied herein is intended, or will be construed, to confer upon or give any person other than the Parties hereto, and their successors or permitted assigns, any right, remedy, obligation or liability under or by reason of this Agreement, or result in such person being deemed a third-party beneficiary of this Agreement. |
| 18.13 | No Strict Construction; Headings; Interpretation. This Agreement has been prepared jointly and will not be strictly construed against any Party. This Agreement contains headings only for convenience and the headings do not constitute or form a part of this Agreement, and should not be used in the construction of this Agreement. The words “include,” “includes” and “including” when used in this Agreement are deemed to be followed by the phrase “but not limited to.” |
| 18.14 | Counterparts. This Agreement may be executed in any number of counterparts, each of which will be deemed to be an original and all of which together will constitute one and the same instrument. An executed counterpart of this Agreement (the entire Agreement, not just a signature page) may be delivered by e-mail (in PDF or another agreed format). |
| 18.15 | Force Majeure. No Party shall be liable to any other for any delay or non-performance of its obligations under this Agreement arising from any Force Majeure Event. “Force Majeure Event” means any act or event, in whole or in part, whether foreseen or unforeseen, that is beyond the reasonable control of a Party, but excludes economic hardship or insufficiency of funds. |
| Page 20 of 38 Confidential |
| 18.16 | Academic Freedom. Subject to the obligations herein, nothing in this Agreement will limit or prohibit Institution or any of its personnel, including the Investigator, from conducting any research or from performing research for or with any entity or person, including any other outside sponsors. GSK and LIXTE acknowledges that this provision is intended to preserve the academic freedom and integrity of Institution and its faculty and to ensure that Institution and its faculty are not regarded as exclusive researchers for GSK or LIXTE. |
| 18.17 | Subordination to Applicable Law. The Parties will not be required to perform any act or to refrain from any act that would violate any law. This Agreement is subject to, and the Parties agree to comply with, all applicable laws. Any provision of any law, statute, rule or regulation that invalidates any provision of this Agreement, that is inconsistent with any provision of this Agreement, or that would cause one or any of the Parties hereto to be in violation of law will be deemed to have superseded the terms of this Agreement. The Parties, however, will use all reasonable endeavors to accommodate the terms and intent of this Agreement to the greatest extent possible consistent with the requirements of the law and will negotiate in good faith toward amendment of this Agreement in such respect. If the Parties cannot reach agreement on an appropriate amendment, then this Agreement may be immediately terminated by either Party. |
| 18.18 | Notice of Texas State Agency. Institution is an agency of the State of Texas and under the Constitution and the laws of the State of Texas possesses certain rights and privileges, is subject to certain limitations and restrictions, and only has such authority as is granted to it under the Constitution and laws of the State of Texas. Notwithstanding any provision hereof, nothing in this Agreement is intended to be, nor will it be construed to be, a waiver of the sovereign immunity of the State of Texas or a prospective waiver or restriction of any of the rights, remedies, claims, and privileges of the State of Texas. Moreover, notwithstanding the generality or specificity of any provision hereof, the provisions of this Agreement as they pertain to Institution are enforceable only to the extent authorized by the Constitution and laws of the State of Texas; accordingly, to the extent any provision hereof conflicts with the Constitution or laws of the State of Texas or exceeds the right, power or authority of Institution to agree to such provision, then that provision will not be enforceable against Institution or the State of Texas. |
[Signature page to follow]
| Page 21 of 38 Confidential |
IN WITNESS WHEREOF, this Agreement is executed as of the Effective Date by a duly authorized representative of each of GSK, LIXTE and Institution.
| GLAXOSMITHKLINE LLC | Lixte Biotechnology Holdings. Inc. | |||
| By: | /s/ Marc Harris | By: | /s/ John S. Kovach | |
| Print Name: | Marc Harris | Print Name: | John S. Kovach, MD | |
| Title: | Assoc Director | Title: | CEO | |
| Date: | 18-Sep-2023 | Date: | Sep 18, 2023 | |
|
The University of Texas M. D. Anderson Cancer Center |
||||
| By: | /s/ Amy M Moritz | |||
| Print Name: | Amy M Moritz | |||
| Title: | Assistant Director, ORA | |||
Date: |
8/28/2023 | |||
| Read and Acknowledged: | ||||
| INVESTIGATOR | ||||
| /s/ Amir Jazaeri | ||||
| Print Name: | Amir Jazaeri, MD | |||
| 8/28/2023 | ||||
| Page 22 of 38 Confidential |
Appendix A
DELINEATION OF GSK AND lixte INVESTIGATIONAL PRODUCT (“IP”) RESPONSIBILITIES
| Institution Contact for IP | Investigational Pharmacy Services INVdrugs@mdanderson.org | |
|
GSK Contact for IP |
Tyler Lockard , Study Delivery Lead external.research@gsk.com | |
| LIXTE Contact for IP | Eric Forman eforman@lixte.com |
|
GSK IP LIXTE IP |
Investigational Dostarlimab (50mg/mL (10mLvial)) Investigational LB-100 (1 mg/mL (10mL vial) |
|
| Number of Study Subjects | Twenty-one (21) (includes Northwestern Study Subjects) | |
| Average Number of Cycles/Subject | 14 | |
| Participating Country | US | |
| Number of Participating Sites | 2 (two) | |
| Study Type |
☒ Open: Identity of the Investigational Product is not withheld from the investigator or subjects at the time of dispensing
☐ Blind: The investigator, pharmacist, and subjects are not able to distinguish between treatment groups at time of dispensing*
☐ Third-party Blind: The investigator and subjects are not able to distinguish between treatment groups, but pharmacy staff will have access to the identity of the IP at the time of dispensing |
| Page 23 of 38 Confidential |
| Study Set Up |
Institution |
GSK or qualified delegate for GSK IP Only | LIXTE or qualified delegate for LIXTE IP Only | |||
| Description of IP provided to country regulatory authorities, and provision of cross-reference letter for use by Institution | X | X | ||||
| Submission to regulatory authority, as appropriate (Clinical Trial Application or IND. | X | |||||
| Supply of IP with appropriate labeling | X | X | ||||
| Additional labeling to comply with applicable local, legal and regulatory requirements, if necessary | X | |||||
| Retention samples of IP | X | X |
| IP Set Up |
Institution |
GSK or qualified delegate for GSK IP Only |
LIXTE or qualified delegate for LIXTE IP Only | |||
| Collection of regulatory approvals and submission of Institution’s and Participating Site’s initial shipment approval checklist to GSK and LIXTE | X |
| Shipment Strategy | Institution | GSK or qualified delegate for GSK IP Only | LIXTE or qualified delegate for LIXTE IP Only | |||
| Shipment direct to Institution and Participating Site(s) | X | X |
| IP Management | Institution | GSK or qualified delegate for GSK IP Only | LIXTE or qualified delegate for LIXTE IP Only | |||
| Provide Institution /Investigator with expiry date, storage conditions, and allowable excursions in a storage and handling manual | X | X | ||||
| Provide GSK & LIXTE with acknowledgement of receipt of all IP shipments to Participating Site(s) | X | |||||
| Monitor expiry date, comply with storage conditions, and report temperature excursions | X | |||||
| Assessment of potential quality issues which occur during shipment or storage of IP to Participating Site(s) | X | X | ||||
| Report inventory use and resupply IP requirements as specified by GSK or LIXTE, including real time use of IRT/RTSM system, as applicable. | X | |||||
| Decision to recall | X | X | ||||
| Execution of recall(s), if applicable, including communication of recall to Participating Site(s) | X | |||||
| Providing recall communication and reporting compliance of recall to GSK and LIXTE | X | |||||
| Destruction of IP (at end of study or recall) and written memo confirming destruction of IP at Participating Site(s) | X |
| Page 24 of 38 Confidential |
SCHEDULE 1 TO APPENDIX A
SUPPLIES AGREEMENT FORM
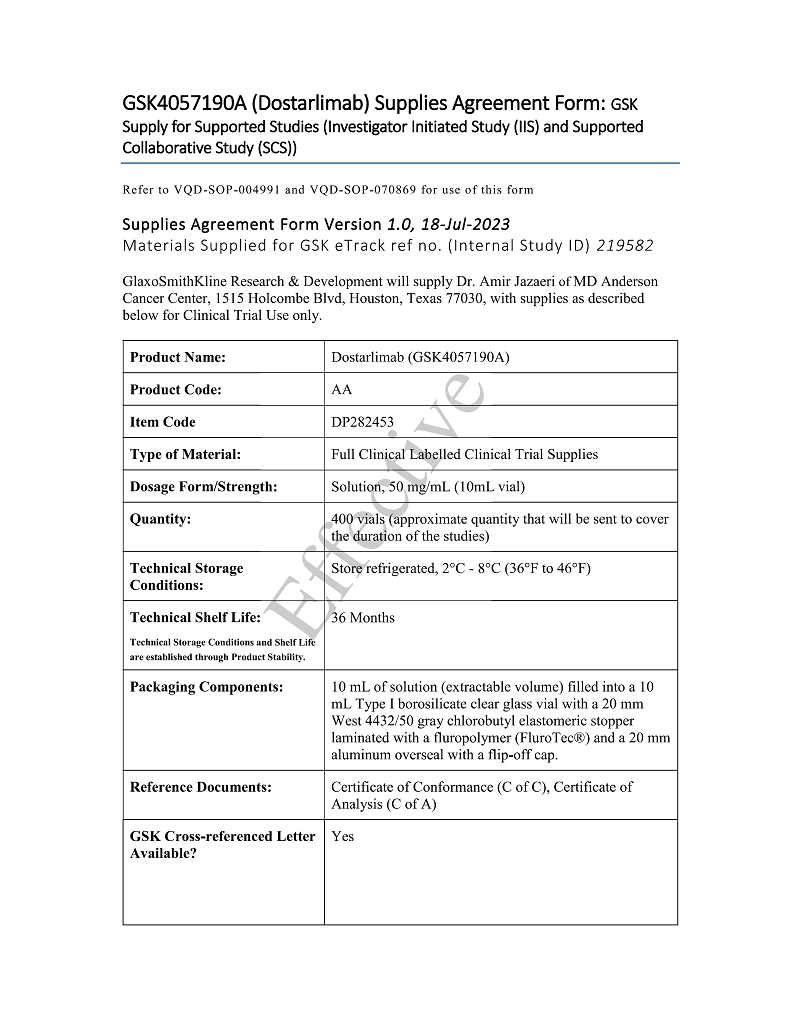
| Page 25 of 38 Confidential |

| Page 26 of 38 Confidential |
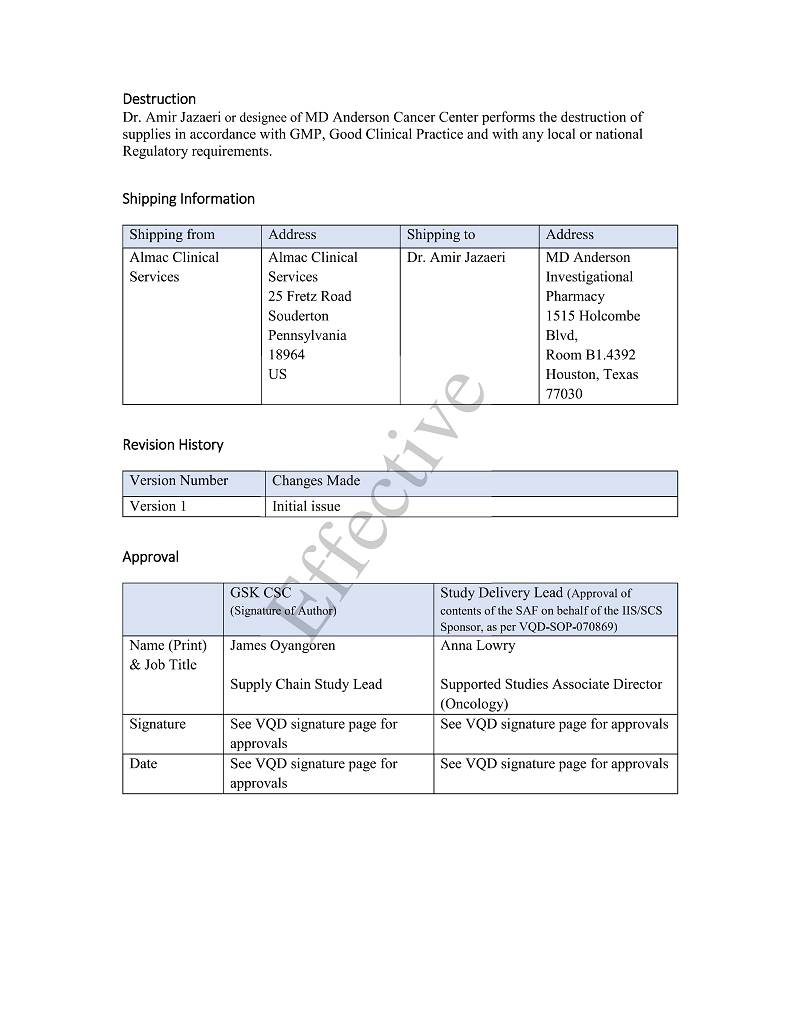
| Page 27 of 38 Confidential |
| Page 28 of 38 Confidential |
APPENDIX B: FUNDING
| Institution: | The University of Texas MD Anderson Cancer Center | |
| Investigator: | Dr. Amir A.Jazaeri | |
| GSK Investigational Product: | Dostarlimab | |
| GSK Protocol # : | 219582 |
| 1. | Enrollment of Study Subjects |
Institution will enroll (which, for clarity, does not include any screening failures) a maximum of twenty-one (21) Study subjects (includes Study Subjects at Northwestern) on the Protocol and use reasonable efforts to achieve an expected rate of 2 Study subjects/month. GSK will pay Institution for Study subjects enrolled on the Protocol that receive at least one dose of GSK Investigational Product and LIXTE Investigational Product, in accordance with the schedule below.
| 2. | Payment Schedule |
GSK agrees to provide Funding in support of the conduct of the Study in the total amount of $1, 493,019.40 USD. Funding will be provided as follows:
| Milestone | $ Payment USD | |||
| Upon receipt by GSK of (i) the final and fully executed Agreement, (ii) all required documentation, and, (iii) confirmation that the summary Protocol has been posted on clinicaltrials.gov or other public register in accordance with the Agreement. | 160,345.04 | |||
| Upon enrollment of 1 (one) Study subject. | 172,345.08 | |||
| Upon enrollment of 4 additional (four) Study subjects (total 5). | 172,345.08 | |||
| Upon enrollment of 4 additional (four) Study Subjects (total 9). | 172,345.08 | |||
| Upon enrollment of 4 additional (four Study Subjects (total 13). | 172,345.08 | |||
| Upon enrollment of 4 additional (four) Study Subjects (total 17). | 172,345.08 | |||
| Upon enrollment of 4 additional (four) Study Subjects (total 21). | 172,345.08 | |||
| Upon receipt of documentation that (i) 21 Study Subjects have completed the Study; (ii) GSK’s receipt of the Final Report or draft manuscript in accordance the Communication of Data Section of this Agreement; (iii) confirmation that final Results summary has been posted to www.ClinicalTrials.gov or other public register in accordance with the Publication Section of this Agreement; and (iv) documentation of attempt of publication. If the Investigator and GSK agree that the Study Results do not support a publication, a written final study report may be accepted for final payment. | 298,603.88 | |||
TOTAL |
1,493,019.40 | |||
| Page 29 of 38 Confidential |
| 3. | Invoicing |
To ensure timely payment, all invoices will be submitted to Tyler Lockard (external.research@gsk.com) (“SAP”) in accordance with the invoice instructions below. GSK will pay Institution within thirty (30) days from GSK receipt of invoice. Institution shall create and submit all its invoices and/or credit notes and sent through email to the SAP. Each Invoice will have to indicate the Purchase Order number, that will be communicated by GSK after contract execution, as well as the following information:
| i. | Invoice addressed to: GSK LLC, 1250 South Collegeville Road, Collegeville, PA., 19426 | |
| ii. | Institution Name/address: | |
| iii. | Study Title: 219582 | |
| iv. | CID: 574189 | |
| v. | Invoice number & date: | |
| vi. | Purchase Order Number: | |
| vii. | GSK Contact: Tyler Lockard | |
| viii. | VAT ID number | |
| ix. | Detailed description of services/milestone: | |
| x. | Bank account name/address & Sort Code |
Failure to provide the required information will delay approval and the invoice may be returned for revisions.
Payment Instructions:
Payments shall be made by Electronic Funds Transfer via the Automated Clearing House (ACH), which is Institution’s preferred method to receive payments.
FOR ACH DELIVERY
Bank Routing Number: 111000614; Account Number: 522292058
Account Name: Univ. of Texas MD Anderson Cancer Center-Office of Grants and Contracts
| 4. | Reconciliation |
In the event that the Study closes enrollment prior to achievement of the maximum number of Study subjects, Institution shall provide written notice to GSK of the date the Study was closed, the proposed funding reconciliation, the number of Study subjects that are eligible for payment in accordance with the terms in Section 1, and any other relevant documentation supporting the plan. GSK will review the proposed reconciliation and will respond with any objections.
The Payment Schedule described in Section 2 will be prorated up to a maximum of:
| Description | Per Unit Cost (USD) | # Units | $ Total USD | |||||||||
| One-time start-up fees | 160,345.04 | |||||||||||
| Per Study subject costs | 63,460.67 | 21 | 1,332,674.06 | |||||||||
| Total | 1,493,019.10 | |||||||||||
| Page 30 of 38 Confidential |
Appendix C: TASK RESPONSIBILITY MATRIX
| TASK | GSK | LIXTE | Institution | |||
| Budget | ||||||
| Make payments according to milestones agreed in contract | X | |||||
| Develop itemised budget | X | |||||
| Study Start Up | ||||||
| Site/Investigator selection & training | X | |||||
| Scientific exchange on research proposal | X | X | X | |||
| Create study documents (Protocol, ICF, Statistical Plan, CRF, Diary Cards, and Laboratory Manual etc.) | X | |||||
|
Contribute to study documents (review and comment)
NB type of study documents to be detailed
Study Protocol, ICF(s), Pharmacy Manual, GSK /LIXTE Investigational Product Handling Instructions, Laboratory Manual
GSK /LIXTE also to contribute for comments received by CA / EC as required and especially in relation to the GSK Investigational Product and LIXTE Investigational Product |
X | X | X | |||
| Service Provider selection and contract, including laboratories if applicable | X | |||||
| Regulatory (e.g. IND application or amendment) | X | |||||
| Ethics Committee/Institutional Review Board Submission | X | |||||
| Protocol Summary posting on ClinicalTrials.gov | X | |||||
| Study Conduct | ||||||
| GSK and LIXTE to provide respective IP to Participating Site (s), upon Institution providing shipping details (address and name) to GSK and LIXTE | X | X | ||||
| Conduct study in adherence with applicable GxP and regulatory requirements | X | |||||
| Study site management (e.g., Monitoring visit, Investigator site file, etc.) | X | |||||
| Ensure sites use GSK’s IRT system in real-time to enroll patients, dispense drug and manage drug study | X | |||||
| Perform Quality Assurance site audits | X | |||||
| Updates to the Investigator’s Brochure for respective IP | X | X | ||||
| Data collection and data management | X | |||||
| Human Biological Sample Management | X | |||||
| Storage of samples as per Study Protocol for future research and development | X | |||||
| Report Clinical Safety Information (SAE & pregnancy initial and follow-up) to GSK and LIXTE | X | |||||
| Transfer of samples to GSK and/or LIXTE or preferred vendor for future research and development if required | x | |||||
| Submit safety report to ECs/IRBs/regulatory authorities | X | |||||
| Provide on a quarterly basis a line listing of all SAE and pregnancies received during a defined quarter to GSK. These listings should be sent to the following email address: PV.ICSRManagement@gsk.com | X | |||||
| Perform reconciliation and feedback to Institution on quarterly basis upon line listing receipt | X | X | ||||
| Perform audit activities of Institution activities | X | X | ||||
| STUDY CLOSURE & ARCHIVING | ||||||
| Conduct Participating Site Close out activities including product reconciliation | X | |||||
| Perform Data Management and Statistical Analysis | X | |||||
| Provide Data to GSK and to LIXTE | X | |||||
| Create Study Report and Manuscript | X | |||||
| Review Study Report and Manuscript | X | x | ||||
| Contribute (review and comment) to publications (abstracts, presentations, CSR, Manuscripts) | X | x | ||||
| Disclosure of Study Results Summary and submission of manuscripts | x | |||||
| Notification of Study end to regulatory authorities and ECs/IRBs | x | |||||
| Archiving of study files | x | |||||
| Page 31 of 38 Confidential |
APPENDIX D
Safety and Efficacy of Targeting PP2A in Ovarian Clear Cell Carcinoma (OCCC) using Dostarlimab and LB-100
PROTOCOL PROVIDED SEPARATELY AND REFERRED TO IN THIS AGREEMENT AS IF SET FORTH IN FULL
| Page 32 of 38 Confidential |
APPENDIX E
ANTICIPATED TIMELINES
| Milestone |
Target Date (dd/mon/year) |
|
| Final Protocol approved | 07/JUN/2023 | |
| Ethics Committee(EC) / Competent Authority (CA) submission | 21/JUN/2023 | |
| EC /CA approval | 01/AUG/2023 | |
| First Subject, First Visit | 01/OCT/2023 | |
| Last Subject, First Visit | 01/AUG/2025 | |
| Last Subject, Last Visit | 01/AUG/2026 | |
| Database Freeze | 01/AUG/2027 | |
| Final Report (or draft manuscript) delivered to GSK in accordance with Clause 4.1 | 01/JAN/2028 | |
| Manuscript in accordance with Clause 4.1 submitted for publication within 90 days of Study completion at all Study Site(s) | 01/APR/2028 |
| Page 33 of 38 Confidential |
APPENDIX F:
Safety Language for Supported Studies GSK Investigational Product
Institution/Investigator Obligations
Under GCPs, applicable laws, and terms of this Agreement, Institution is responsible for and undertakes to assess all clinical safety information arising during the Study in order to generate all safety reports as required by applicable laws. Such safety reports will include, but may not be limited to, Individual Case Safety Reports (“ICSRs”) for Suspected Unexpected Serious Adverse Reactions (“SUSARs”) and, where applicable, Development Safety Update Report(s) (“DSURs”). Institution is responsible for submitting such reports to all concerned regulatory authorities, relevant Independent Ethics Committee(s) (“IEC”) or Institutional Review Board IRB(s) and individual Study investigator(s), as required, and within applicable timelines.
In the event that GSK maintains its own IB(s) for the GSK IMP being investigated under the Study, regardless of the indication under study, GSK will provide these IB(s), and any updates, and/or supplements to these IB(s) to Institution during the course of the Study for information purposes. Institution shall communicate the IB to the Investigator and Sub-investigators during the course of the Study for information purposes.
If any GSK IMP being investigated under the Study are marketed products, or become marketed products during the Study, Institution shall be responsible for providing to the Investigator, Participating Site(s), and Sub-investigator(s) access the current approved local country product information in respect of the marketed GSK IMP through whatever means available to health care professionals in the countries where the Study will be conducted (e.g., internet repositories, published compendia/formularies, etc.).
In the event that GSK produces any of its own DSURs in respect of the GSK IMP, GSK will provide to Institution on request and for the duration of the Study, copies of the executive summary and any line listings of serious adverse reactions extracted from approved GSK DSURs for information only and to assist Institution in the generation of its own DSUR(s), where applicable. Investigator and Institution agrees not to forward such GSK DSUR sections to any third party.
GSK will ensure that any urgent safety issues relating to the GSK IMP provided for the Study will be communicated to the Institution by whatever means that GSK, in its sole discretion, deems appropriate. The Institution shall communicate such issues to the Investigator, Participating Site(s), and Sub-investigator(s) during the course of the Study.
Forms
Institution will use its internal form for SAE reporting and GSKs forms for pregnancy reporting.
Case Exchange
| a. | Investigator shall report all SAEs arising during the Study in Study Subjects exposed to the GSK IMP (as defined by the Protocol), to GSK (as specified below) using an approved form within twenty-four (24) hours or latest one (1) business day of first becoming aware of the event, regardless of Investigator/designee causality assessments against GSK IMP. |
| Page 34 of 38 Confidential |
Pregnancy Information
| b. | Investigator will report pregnancy information on any female Study Subject who becomes pregnant while participating in the Study and following exposure to a GSK IMP, to GSK (as specified below) using an approved form within 24 hours or latest one (1) business day of first becoming aware of the pregnancy. The Study Subject will also be followed to determine the outcome of the pregnancy (including any premature termination of the pregnancy). Information on the status of the mother and child will be forwarded to GSK. Generally, follow-up will be requested by GSK no longer than six (6) to eight (8) weeks following the estimated delivery date. |
Note:
There are two forms – initial and follow-up.
Reporting Clinical Safety Information to GSK
| c. | In this Study, there is the potential for the unsolicited reporting of GSK-product-related events, by a patient, to the Investigator, and/or there is the potential that the Investigator may read of GSK-product-related events in a patient’s medical records. Any events (AE, SAE, or pregnancies) considered to be related to a GSK product, that occur during the Study, need to be reported according to country regulatory guidelines. Study participation-related events should be collected and reported according to country regulatory guidelines. |
Reporting Period
| d. | The SAEs and pregnancy reports that are subject to the above reporting provisions are those that occur following the first dose of the GSK IMP as long as Protocol defines. |
Requesting Follow-up Information
| e. | Investigator will provide GSK with details of whom GSK shall address requests for follow up information on SAE and pregnancy reported from this Study, and further agrees to update such contact details as necessary. At the time of this Agreement, all such requests should be addressed to: |
Dr. Amir Jazaeri (aajazaeri@mdanderson.org)
Investigator shall submit to GSK (as specified above) such further detailed information relating thereto as GSK shall request within twenty-four (24) hours or latest one (1) business day of it becoming available.
Events Exempt from Reporting to GSK
| f. | Any blinded ICSRs or any unblinded reports for Study Subjects exposed only to placebo or a non-GSK comparator during the Study. |
Routing of Clinical Safety Data to GSK
| g. | Notwithstanding the Force Majeure Clause of this Agreement, such reports and information as outlined above, including Investigator causality assessments against all concerned GSK IMP and English translations where reporting is from a non-English speaking country, shall be sent via email to the below destination: |
Pharma:
OAX37649@GSK.com
| Page 35 of 38 Confidential |
Reconciliations
If SCS: Quarterly reconciliations shall be performed.
Communication for reconciliation shall be sent via email to the below destination:
Reconciliation mailbox: Pharma: PV.ICSRManagement@gsk.com
Definitions
Adverse Event (AE) – Any untoward medical occurrence in a patient or clinical trial subject administered a medicinal product and which does not necessarily have a causal relationship with this treatment. An adverse event can therefore be any unfavourable and unintended sign (e.g., an abnormal laboratory finding), symptom, or disease temporally associated with the use of a medicinal product, whether or not considered related to the medicinal product.
Adverse Events of Special Interest (AESI) - An adverse event of special interest (serious or non-serious) is one of scientific and medical concern specific to the Institution’s product or program, for which ongoing monitoring and rapid communication by the investigator to the Institution can be appropriate. Such an event might warrant further investigation in order to characterize and understand it. Depending on the nature of the event, rapid communication by the trial Institution to other parties (e.g., regulators) might also be warranted.
Development Safety Update Report (DSUR) – A periodic reporting on drugs under development (see ICH-E2F Guideline, Volume 10 of the Rules Governing Medicinal Products in the EU).
Emerging Safety Issue - A safety issue considered by a marketing authorisation holder to require urgent attention by the competent authority because of the potential major impact on the risk-benefit balance of the medicinal product and/or on patients’ or public health and the potential need for prompt regulatory action and communication to patients and healthcare professionals. Examples include: major safety issues identified in the context of ongoing or newly completed studies, e.g., an unexpectedly increased rate of fatal or life-threatening adverse events; major safety issues identified through the spontaneous reporting system or publications in the scientific literature, which may lead to considering a contraindication, a restriction of use of a medicinal product or its withdrawal from the market; major safety-related regulatory actions outside the EU, e.g., a restriction of use of a medicinal product or its suspension.
Investigational New Drug Safety Report (INDSR) - Is a written safety report used by Institutions to notify FDA of any adverse experience associated with the use of the drug that is both serious and unexpected.
Investigator’s Brochure (IB) - A compilation of the clinical and nonclinical data on the investigational product(s) which is relevant to the study of the investigational product(s) in human subjects.
Pregnancy Cases - Cases originating from spontaneous or clinical trial sources. Pregnancy data is any abnormal pregnancy, normal pregnancy outcome or adverse event/special situation following direct exposure to a GSK product, via a patient’s partner, or via breast milk (lactation exposure).
| Page 36 of 38 Confidential |
Pregnancy/Lactation Exposure - With or without any AEs related to the parent or child. Use of a product while pregnant and/or breastfeeding.
Reference Safety Information (RSI) - In periodic benefit-risk evaluation reports for medicinal products, all relevant safety information contained in the reference product information (e.g., the company core data sheet) prepared by the marketing authorisation holder and which the marketing authorisation holder requires to be listed in all countries where it markets the product, except when the local regulatory authority specifically requires a modification (see GVP Annex IV, ICH-E2C(R2) Guideline)”
Serious Adverse Event (SAE) - An untoward medical occurrence that at any dose: results in death, is life-threatening (NOTE: The term “life-threatening” in the definition of “serious” refers to an event in which the patient was at risk of death at the time of the event; it does not refer to an event which hypothetically might have caused death if it were more severe), requires inpatient hospitalisation or prolongation of existing hospitalization, results in persistent or significant disability/incapacity, is a congenital anomaly/birth defect, is a medically important event or reaction
Signal - Information arising from one or multiple sources, including observations and experiments, which suggests a new potentially causal association, or a new aspect of a known association between an intervention and an event or set of related events, either adverse or beneficial, that is judged to be of sufficient likelihood to justify verificatory action.
Suspected Unexpected Serious Adverse Reaction (SUSAR) - All suspected adverse reactions related to an IMP (the tested IMP and comparators) which occur in the concerned trial that are both unexpected and serious (SUSARs) are subject to expedited reporting.
Terms of Interest (TOI) - A group of MedDRA terms maintained in the Integrated Coding Dictionary System (ICDS). TOIs may be comprised of levels of the MedDRA hierarchy at the Preferred Term (PT) level or higher. TOIs may also be constructed from Standardized MedDRA Queries (SMQs), other TOIs in a ‘building-block’ manner or contain mixtures of TOIs and levels of the MedDRA hierarchy.
| Page 37 of 38 Confidential |
APPENDIX G
Institution Investigational Pharmacy Services (IPS) Requirements
| 1. | Minimum Labeling Standards |
Product Labeling by GSK and LIXTE
| A. | GSK and LIXTE shall ensure that all immediate containers of their respective Investigational Product must, at a minimum, be labeled with the following information: |
| i. | Name of product | |
| ii. | Lot or batch number | |
| iii. | Storage conditions | |
| iv. | Quantity | |
| v. | Formulation | |
| vi. | Name and address of manufacturer or GSK or LIXTE, as applicable |
Note: Investigational Product that is shipped to Institution and not labeled as described above will be deemed unacceptable for use and will be destroyed or returned to GSK or LIXTE, as applicable, at its cost.
| 2. | Drug Expiration/Re-Test Dating Information |
GSK and LIXTE shall ensure that Re-test and/or expiration dating information shall be provided to Institution with each lot of their respective Investigational Product. Investigational Product may be quarantined by Institution until such information is provided.
| Page 38 of 38 Confidential |