10-K: Annual report [Section 13 and 15(d), not S-K Item 405]
Published on March 24, 2025
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM
| ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
| For
the fiscal year ended |
| TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
| For the transition period from ______ to ______ |
Commission
file number:
(Exact name of registrant as specified in its charter)
| (State or other jurisdiction of | (I.R.S. Employer | |
| incorporation or organization) | Identification Number) | |
| (Address of principal executive offices) | (Zip Code) |
Registrant’s
telephone number: (
Securities registered pursuant to Section 12(b) of the Act: Common Stock, $0.0001 par value.
| Title of each class | Trading Symbol(s) | Name of each exchange on which registered | ||
| The Stock Market LLC | ||||
| The Stock Market LLC |
Indicate
by check mark if the registrant is a well-known seasoned issuer as defined in Rule 405 of the Securities Act. Yes ☐
Indicate
by check mark if the registrant is not required to file reports pursuant to Section 13 or 15(d) of the Exchange Act. Yes ☐
Indicate
by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange
Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2)
has been subject to such filing requirements for the past 90 days.
Indicate
by check mark whether the registrant has submitted electronically every Interactive Data File required to be submitted pursuant to Rule
405 of Regulation S-T (§ 232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant
was required to submit such files).
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, a smaller reporting, or emerging growth company. See the definitions of “large accelerated filer,” “accelerated filer,” “smaller reporting company,” and “emerging growth company” in Rule 12b-2 of the Exchange Act.
| Large accelerated filer | ☐ | Accelerated filer | ☐ |
| ☒ | Smaller reporting company | ||
| Emerging growth company |
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☐
Indicate
by check mark whether the registrant has filed a report on and attestation to its management’s assessment of the effectiveness
of its internal control over financial reporting under Section 404(b) of the Sarbanes-Oxley Act (15 U.S.C. 7262(b)) by the registered
public accounting firm that prepared or issued its audit report.
If
the securities are registered pursuant to Section 12(b) of the Act, indicate by check mark whether the financial statements of the registrant
included in the filing reflect the correction of an error to previously issued financial statements.
Indicate by check mark whether any of those error corrections are restatements that required a recovery analysis of incentive-based compensation received by any of the registrant’s executive officers during the relevant recovery period pursuant to §240.10D-1(b). ☐
Indicate by check mark whether registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act):
Yes
☐ No
The
aggregate market value of the common stock held by non-affiliates of the registrant as of June 30, 2024 was approximately $
The Company had shares of common stock issued and outstanding as of March 14, 2025.
Documents
incorporated by reference:
TABLE OF CONTENTS
| -2- |
Introductory Comment
Throughout this Annual Report on Form 10-K, the terms “we,” “us,” “our,” “our company,” “Lixte,” the “Company” and the “Registrant” refer to Lixte Biotechnology Holdings, Inc., a Delaware corporation, and Lixte Biotechnology, Inc., a Delaware corporation, our wholly-owned subsidiary.
FORWARD-LOOKING STATEMENTS
This Annual Report on Form 10-K (the “Report”) contains certain forward-looking statements. For example, statements regarding our financial position, business strategy and other plans and objectives for future operations, and assumptions and predictions about future product demand, supply, manufacturing, costs, marketing and pricing factors are all forward-looking statements. These statements are generally accompanied by words such as “intend,” “anticipate,” “believe,” “estimate,” “potential(ly),” “continue,” “forecast,” “predict,” “plan,” “may,” “will,” “could,” “would,” “should,” “expect” or the negative of such terms or other comparable terminology. We believe that the assumptions and expectations reflected in such forward-looking statements are reasonable, based on information available to us on the date hereof, but we cannot assure you that these assumptions and expectations will prove to have been correct or that we will take any action that we may presently be planning. However, these forward-looking statements are inherently subject to known and unknown risks and uncertainties. Actual results or experience may differ materially from those expected or anticipated in the forward-looking statements. Factors that could cause or contribute to such differences include, but are not limited to, regulatory policies, competition from other similar businesses, and market and general policies, competition from other similar businesses, and market and general economic factors. This discussion should be read in conjunction with the consolidated financial statements and notes thereto included in this Report.
If one or more of these or other risks or uncertainties materialize, or if our underlying assumptions prove to be incorrect, actual results may vary materially from what we project. Any forward-looking statement you read in this Report reflects our current views with respect to future events and is subject to these and other risks, uncertainties and assumptions relating to our operations, results of operations, growth strategy, and liquidity. All subsequent forward-looking statements attributable to us or individuals acting on our behalf are expressly qualified in their entirety by this paragraph. You should specifically consider the factors identified in this Report, which would cause actual results to differ before making an investment decision. We are under no duty to update any of these forward-looking statements after the date of this Report or to conform these statements to actual results.
| -3- |
PART I
ITEM 1. BUSINESS
Company Overview
The Company is a clinical-stage biopharmaceutical company focused on identifying new targets for cancer drug development and developing and commercializing cancer therapies. The Company’s product pipeline is primarily focused on inhibitors of protein phosphatase 2A, which is used to enhance cytotoxic agents, radiation, immune checkpoint blockers and other cancer therapies. The Company believes that inhibitors of protein phosphatases have significant therapeutic potential for a broad range of cancers. The Company is focusing on the clinical development of a specific protein phosphatase inhibitor, referred to as LB-100, which has been shown to have clinical anti-cancer activity.
The Company believes that the mechanism by which LB-100 affects cancer cell growth is different from cancer agents currently approved for clinical use. LB-100 is currently being tested in clinical trials in Ovarian Clear Cell Carcinoma, Metastatic Micro Satellite Stable (MSS) Colon Cancer, and Advanced Soft Tissue Sarcoma. LB-100 has shown anti-cancer activity in animal models of glioblastoma multiforme, neuroblastoma, and medulloblastoma, all cancers of neural tissue. LB-100 has also been shown to enhance the effectiveness of commonly used anti-cancer drugs in animal models of melanoma, breast cancer and sarcoma. The enhancement of anti-cancer activity of these anti-cancer drugs occurs at doses of LB-100 that do not significantly increase toxicity in animals. It is therefore hoped that, when combined with standard anti-cancer regimens against many tumor types, LB-100 will improve therapeutic benefit.
As a compound moves through the FDA-approval process, it becomes an increasingly valuable property, but at a cost of additional investment at each stage. As the potential effectiveness of LB-100 has been documented at the clinical trial level, the Company has allocated resources to expand the breadth and depth of its patent portfolio. The Company’s approach has been to operate with a minimum of overhead, moving compounds forward as efficiently and inexpensively as possible, and to raise funds to support each of these stages as certain milestones are reached. The Company’s longer-term objective is to secure one or more strategic partnerships or licensing agreements with pharmaceutical companies with major programs in cancer.
The Company’s activities are subject to significant risks and uncertainties, including the need for additional capital. The Company has not yet commenced any revenue-generating operations, does not have positive cash flows from operations, relies on stock-based compensation for a substantial portion of employee and consultant compensation, and is dependent on periodic access to equity capital to fund its operating requirements.
Description of Business
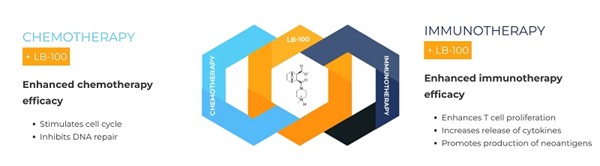
Most cancer patients are treated with either chemotherapy or immunotherapy or both. These therapies often have limited benefit and there is a high unmet medical need to enhance their effects. In many preclinical models we have shown that LB-100 enhances the effect of both chemotherapy and Immunotherapy
| -4- |
LB-100, a small molecule potent inhibitor of PP2A, was designed and developed by us. Numerous preclinical studies have documented that LB-100 potentiates most if not all anti-cancer drugs that damage DNA. LB-100 is not associated with any increase in cytotoxicity when given with cytotoxic drugs. This synergy involves transient interruption of several DNA damage repair pathways by LB-100 and an increase in cell division rate. LB-100 has FDA Investigational New Drug status in the US and Investigational Medicinal Product Dossier approval in the European Union.
In its initial Phase 1 clinical trial, LB-100 given alone daily for 3 days was non-toxic, except for a transient increase in serum creatinine believed to be caused by inhibition of PP2A in the renal tubules. In the Phase 1 clinical trial, the Maximum Tolerated Dose (“MTD”) was 2.33mg/m2 daily for 3 days every 3 weeks. Of the 25 patients with heavily-treated advanced solid tumors with measurable disease, 3 patients had stable disease for 2 cycles, 3 patients had stable disease for 4 cycles, and 3 patients had stable disease for 6 cycles. One patient with pancreatic cancer had a partial response after 12 cycles lasting 534 days.
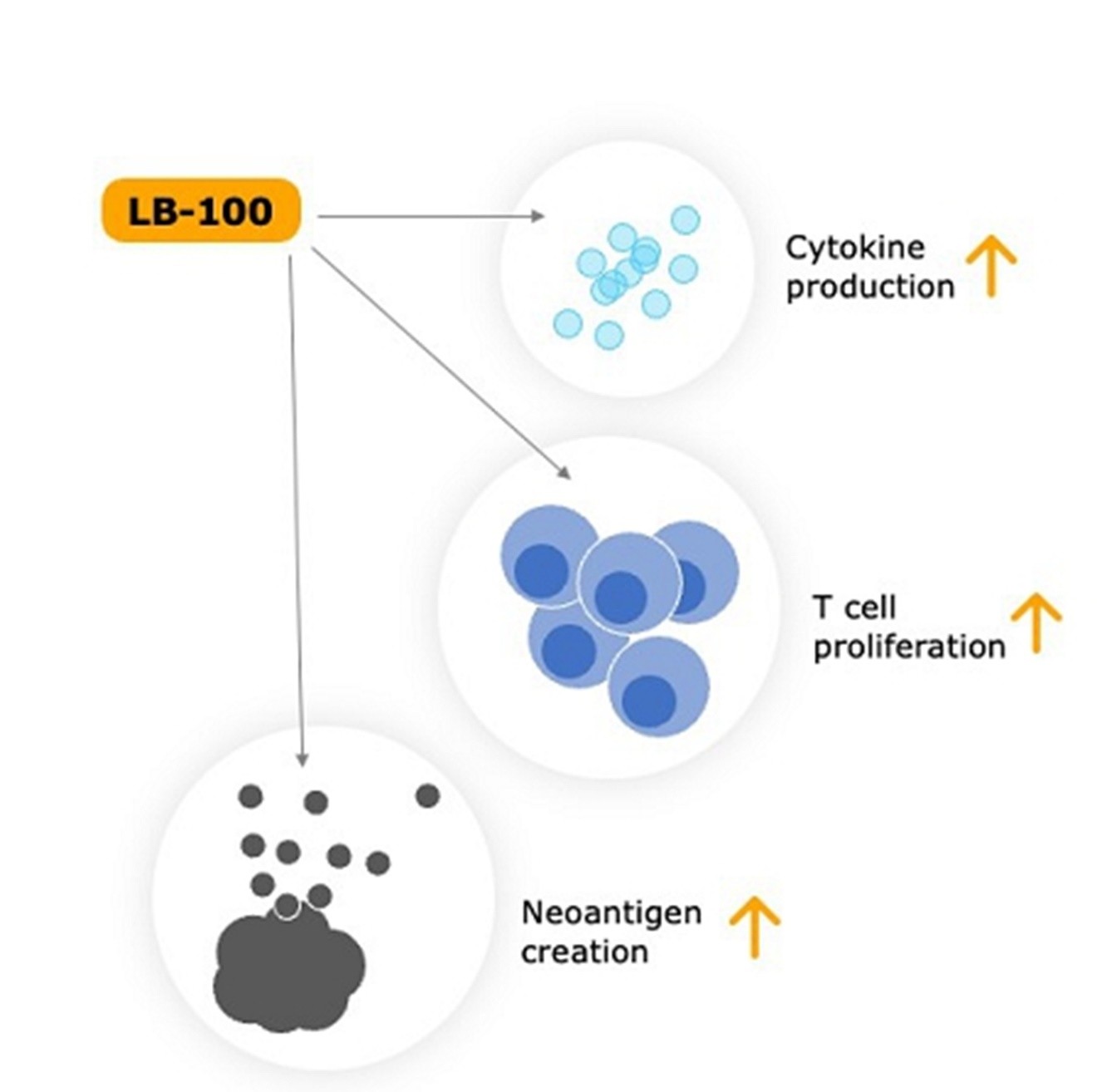
Low doses of LB-100 have now been shown to enhance immune checkpoint inhibition (“ICI”) by several different mechanisms affecting the tumor compartment and immune T-cell compartment. LB-100 increases CD8+T-cell infiltration and CD8-Treg ratio, CD8+T-cell proliferation, and cytokine production induces microsatellite instability, neoantigen production and immune responsiveness, converting immunologically “cold” to “hot” cancers.
| -5- |
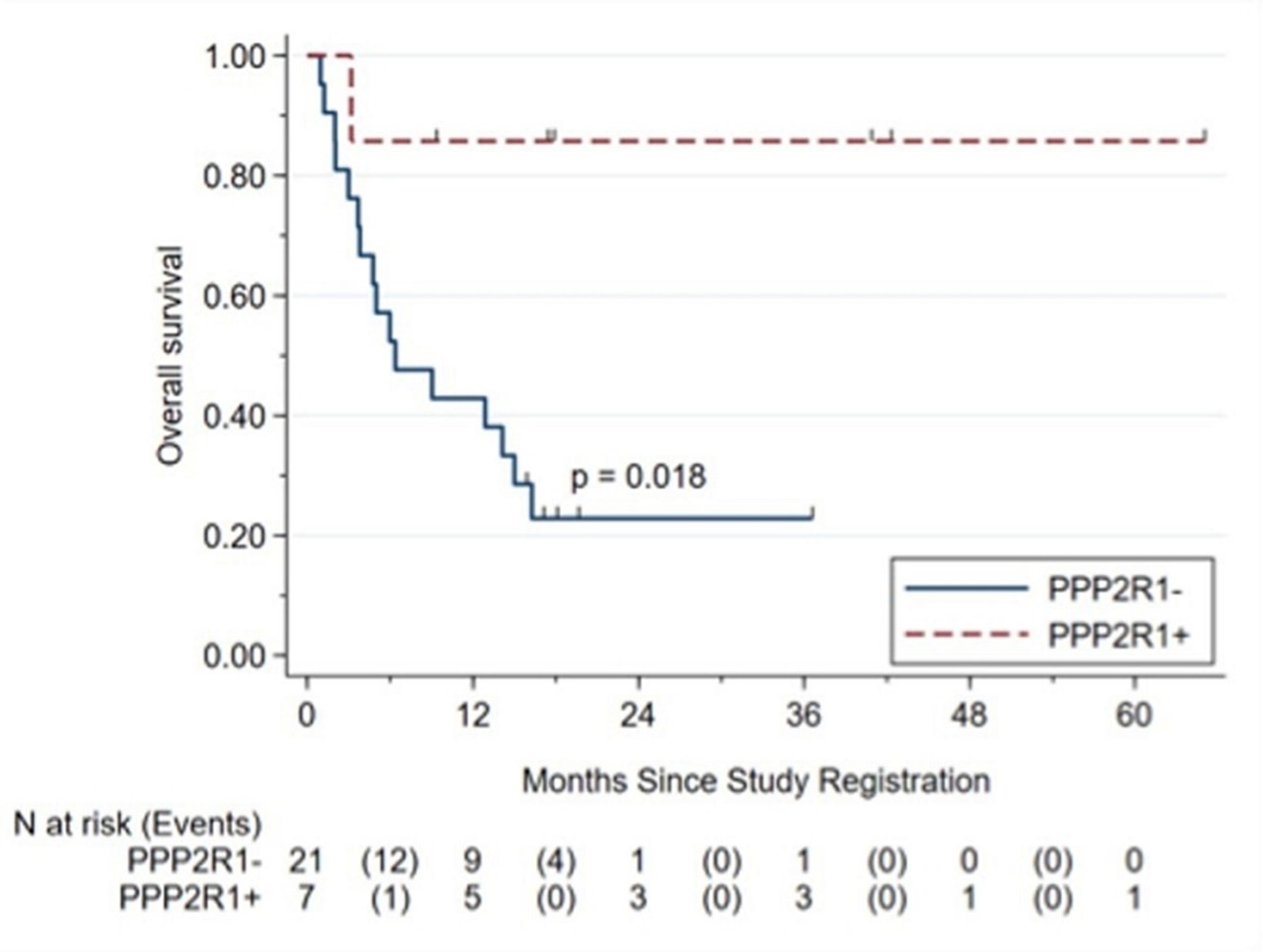
Ovarian clear cell carcinoma patients with inactivating mutations in PPP2R1A, a gene coding for a scaffold component of PP2A, and treated with immune checkpoint inhibitors, were recently found to have markedly longer survival than patients without the mutation in their cancers. Retrospective reviews of patients with a variety of cancers treated with ICI or chemotherapy show much longer survival of ICI-treated patients with a PPP2R1A mutation in their tumors.
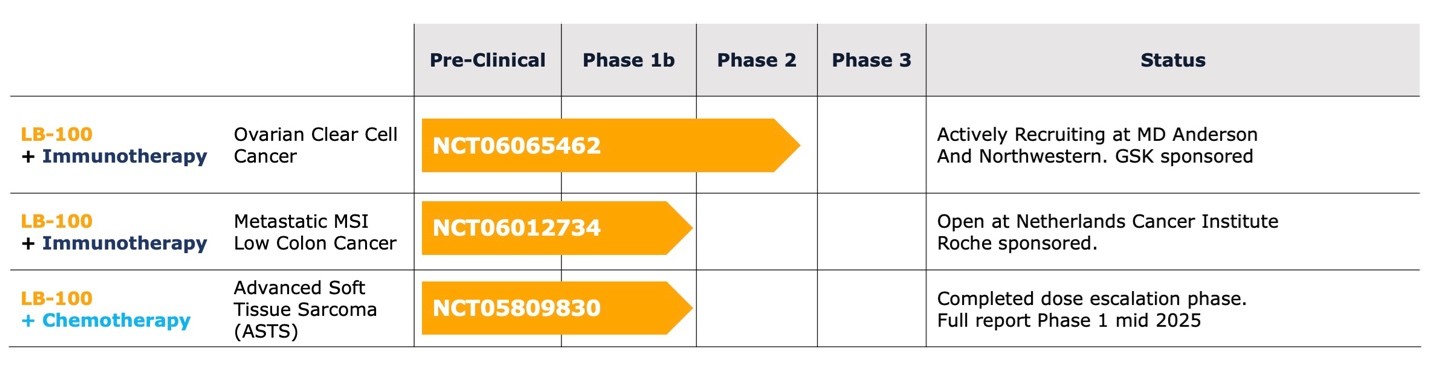
Based on the observations in ovarian clear cell carcinoma, we have initiated a clinical trial in this disease combining LB-100 with a monoclonal antibody blocking PD-1, a protein found on T-cells (NCT06065462).
Given these preclinical and clinical observations, it is likely that LB-100 may be a general way to enhance immunotherapy responses.
| -6- |
The research on the LB-100 series was initiated in 2006 under a Cooperative Research and Development Agreement (“CRADA”) with the National Institute of Neurologic Disorders and Stroke or NINDS of the National Institutes of Health or NIH dated March 22, 2006 that was subsequently extended through a series of amendments until it terminated on April 1, 2013.
We have also designed and developed the LB-200 series, which consists of histone deacetylase inhibitors (HDACi). LB-200 has not advanced to the clinical stage and would require additional capital to fund further development. Accordingly, because of our focus on the clinical development of LB-100 and analogs for cancer therapy as described below in more detail, we have decided not to actively pursue the preclinical development of our LB-200 series of compounds at this time.
Clinical Trial Agreements
Spanish Sarcoma Group Collaboration Agreement
Effective July 31, 2019, we entered into a Collaboration Agreement for an Investigator-Initiated Clinical Trial with the Spanish Sarcoma Group (Grupo Español de Investigación en Sarcomas or “GEIS”), Madrid, Spain, to carry out a study entitled “Randomized phase I/II trial of LB-100 plus doxorubicin vs. doxorubicin alone in first line of advanced soft tissue sarcoma”. The purpose of this clinical trial is to obtain information with respect to the efficacy and safety of LB-100 combined with doxorubicin in soft tissue sarcomas. Doxorubicin is the global standard for initial treatment of advanced soft tissue sarcomas (“ASTS”). Doxorubicin alone has been the mainstay of first line treatment of ASTS for over 40 years, with little improvement in survival from adding cytotoxic compounds to or substituting other cytotoxic compounds for doxorubicin. In animal models, LB-100 consistently enhances the anti-tumor activity of doxorubicin without apparent increases in toxicity.
GEIS has a network of referral centers in Spain and across Europe that have an impressive track record of efficiently conducting innovative studies in ASTS. We agreed to provide GEIS with a supply of LB-100 to be utilized in the conduct of this clinical trial, as well as to provide funding for the clinical trial. The goal is to enter approximately 150 to 170 patients in this clinical trial over a period of two to four years. The Phase 1 portion of the study began in the quarter ended June 30, 2023 to determine the recommended Phase 2 dose of the combination of doxorubicin and LB-100. As advanced sarcoma is a very aggressive disease, the design of the Phase 2 portion of the study assumes a median progression-free survival (“PFS”), no evidence of disease progression or death from any cause) of 4.5 months in the doxorubicin arm and an alternative median PFS of 7.5 months in the doxorubicin plus LB-100 arm to demonstrate a statistically significant decrease in relative risk of progression or death by adding LB-100. There is a planned interim analysis of the primary endpoint when approximately 50% of the 102 events required for final analysis is reached.
On October 13, 2022, we announced that the Spanish Agency for Medicines and Health Products (Agencia Española de Medicamentos y Productos Sanitarios or “AEMPS”) had authorized a Phase 1b/randomized Phase 2 study of LB-100, our lead clinical compound, plus doxorubicin, versus doxorubicin alone, the global standard for initial treatment of advanced soft tissue sarcomas (ASTS). Consequently, this clinical trial commenced during the quarter ended June 30, 2023 and to be completed and a report prepared by December 31, 2026. In April 2023, GEIS completed its first site initiation visit in preparation for the clinical trial at Fundación Jiménez Díaz University Hospital (Madrid). Up to 170 patents will be entered into the clinical trial. The recruitment phase of the Phase 1b portion of the protocol was completed during the quarter ended September 30, 2024. We expect to have data on toxicity and preliminary efficacy from this portion of the clinical trial during the quarter ending December 31, 2025.
Given the focus on the combination of LB-100 with immunotherapy in ovarian clear cell carcinoma and colorectal cancer and the availability of capital resources, the Company entered into Amendment No. 1 to the Collaboration Agreement effective March 11, 2025 that relieved the Company of the financial obligation to support the randomized Phase 2 portion of the clinical trial contemplated in the Collaboration Agreement of approximately $3,095,000. As a result, it is uncertain as to whether the Phase 2 portion of this clinical trial will proceed.
Clinical Research Support Agreement Relating to Small Cell Lung Cancer
| -7- |
We had executed a Clinical Research Support Agreement with the City of Hope National Medical Center to carry out a Phase 1b clinical trial of LB-100 combined with an FDA-approved standard regiment for treatment of untreated extensive-stage disease small cell lung cancer. The clinical trial was initiated on March 9, 2021. However, due to the lack of patient accrual, the Company provided notice to the City of Hope National Medical Center of the Company’s intent to terminate the Clinical Research Support Agreement effective as of July 8, 2024.
MD Anderson Cancer Center Clinical Trial
On September 20, 2023, we announced an investigator-initiated Phase 1b/2 collaborative clinical trial to assess whether adding LB-100 to a human programmed death receptor-1 (“PD-1”) blocking antibody of GSK plc (“GSK”), dostarlimab-gxly, may enhance the effectiveness of immunotherapy in the treatment of ovarian clear cell carcinoma (“OCCC”). The clinical trial is being sponsored by The University of Texas MD Anderson Cancer Center (“MD Anderson”) and is being conducted at The University of Texas - MD Anderson Cancer Center. We are providing LB-100 and GSK is providing dostarlimab-gxly and financial support for the clinical trial. On January 29, 2024, we announced the entry of the first patient into this clinical trial. We currently expect that this clinical trial will be completed by December 31, 2027.
On February 25, 2025, we announced that we had added the Robert H. Lurie Comprehensive Cancer Center (Lurie Cancer Center) of Northwestern University as a second site in a clinical trial combining the Company’s proprietary compound LB-100 with GSK’s dostarlimab to treat ovarian clear cell cancer. Patient recruitment is underway, and the first patient has been dosed.
Netherlands Cancer Institute Clinical Trial
Effective June 10, 2024, we entered into a Clinical Trial Agreement with the Netherlands Cancer Institute (“NKI”) to conduct a Phase 1b clinical trial of the Company’s protein phosphatase inhibitor, LB-100, combined with atezolizumab, a PD-L1 inhibitor, the proprietary molecule of F. Hoffman-La Roche Ltd. (“Roche”), for patients with microsatellite stable metastatic colon cancer. Under the agreement, we will provide our lead clinical compound, LB-100, and under a separate agreement between NKI and Roche, Roche will provide atezolizumab and financial support for the clinical trial. We have no obligation to and will not provide any reimbursement of clinical trial costs. Pursuant to the agreement and the protocol set forth in the agreement, the clinical trial will be conducted by NKI at NKI’s site in Amsterdam by principal investigator Neeltje Steeghs, MD, PhD, and NKI will be responsible for the recruitment of patients. The agreement provides for the protection of the respective intellectual property rights of each of Lixte, NKI and Roche.
This Phase 1b clinical trial will evaluate safety, optimal dose and preliminary efficacy of LB-100 combined with atezolizumab for the treatment of patients with metastatic microsatellite stable colorectal cancer. Immunotherapy using monoclonal antibodies like atezolizumab can enhance the body’s immune response against cancer and hinder tumor growth and spread. LB-100 has been found to improve the effectiveness of anticancer drugs in killing cancer cells by inhibiting a protein called PP2A on cell surfaces. Blocking PP2A increases stress signals in tumor cells expressing the PP2A protein. Accordingly, combining atezolizumab with LB-100 may enhance treatment efficacy for metastatic colorectal cancer, as cancer cells with heightened stress signals are more vulnerable to immunotherapy.
This study comprises a dose escalation phase and a dose expansion phase. The objective of the dose escalation phase is to determine the recommended Phase 2 dose (RP2D) of LB-100 when combined with the standard dosage of atezolizumab. The dose expansion phase will further investigate the preliminary efficacy, safety, tolerability, and pharmacokinetics/dynamics of the LB-100 and atezolizumab combination. The clinical trial opened in August 2024 with the enrollment of the first patient. Patient accrual is expected to take up to 24 months, with a maximum of 37 patients with advanced colorectal cancer to be enrolled in this study.
The principal investigator of the colorectal study testing LB-100 in combination with atezolizumab is currently investigating two Serious Adverse Events (“SAEs”) observed in the clinical trial that was launched in August 2024. The Investigational Review Board (IRB) of the Netherlands Cancer Institute has requested additional information with respect to these SAEs and the study has been paused for enrollment until the IRB’s questions have been, as more fully discussed at “Item 7. Management’s Discussion and Analysis of Financial Condition and Results of Operations – Specific Risks Associated with the Company’s Business Activities – Serious Adverse Events”.
| -8- |
National Cancer Institute Pharmacologic Clinical Trial
In May 2019, the National Cancer Institute (NCI) initiated a glioblastoma (GBM) pharmacologic clinical trial. This study was being conducted and funded by the NCI under a Cooperative Research and Development Agreement, with the Company being required to provide the LB-100 clinical compound.
Primary malignant brain tumors (gliomas) are very challenging to treat. Radiation combined with the chemotherapeutic drug temozolomide has been the mainstay of therapy of the most aggressive gliomas (glioblastoma multiforme or GBM) for decades, with little further benefit gained by the addition of one or more anti-cancer drugs, but without major advances in overall survival for the majority of patients. In animal models of GBM, the Company’s novel protein phosphatase inhibitor, LB-100, has been found to enhance the effectiveness of radiation, temozolomide chemotherapy treatments and immunotherapy, raising the possibility that LB-100 may improve outcomes of standard GBM treatment in the clinic. Although LB-100 has proven safe in patients at doses associated with apparent anti-tumor activity against several human cancers arising outside the brain, the ability of LB-100 to penetrate tumor tissue arising in the brain was not known. Many drugs potentially useful for GBM treatment do not enter the brain in amounts necessary for anti-cancer action.
The NCI study was designed to determine the extent to which LB-100 enters recurrent malignant gliomas. Patients having surgery to remove one or more tumors received one dose of LB-100 prior to surgery and had blood and tumor tissue analyzed to determine the amount of LB-100 present and to determine whether the cells in the tumors showed the biochemical changes expected to be present if LB-100 reached its molecular target. As a result of the innovative design of the NCI study, it was believed that data from a few patients would be sufficient to provide a sound rationale for conducting a larger clinical trial to determine the effectiveness of adding LB-100 to the standard treatment regimen for GBMs. Blood and brain tumor tissue were analyzed from seven patients after intravenous infusion of a single dose of LB-100. Results of the investigation demonstrated that there was virtually no entry of LB-100 into the brain tumor tissue. Accordingly, alternative methods of drug delivery will be required to determine if LB-100 has meaningful clinical anti-cancer activity against glioblastoma multiforme and other aggressive brain tumors.
Patent and License Agreements
National Institute of Health
Effective February 23, 2024, we entered into a Patent License Agreement (the “License Agreement”) with the National Institute of Neurological Disorders and Stroke (“NINDS”) and the National Cancer Institute (“NCI”), each an institute or center of the National Institute of Health (“NIH”). Pursuant to the License Agreement, we have licensed exclusively NIH’s intellectual property rights claimed for a Cooperative Research and Development Agreement (“CRADA”) subject invention co-developed with the Company, and the licensed field of use, which focuses on promoting anti-cancer activity alone, or in combination with standard anti-cancer drugs. The scope of this clinical research extends to checkpoint inhibitors, immunotherapy, and radiation for the treatment of cancer. The License Agreement is effective, and shall extend, on a licensed product, licensed process, and country basis, until the expiration of the last-to-expire valid claim of the jointly owned licensed patent rights in each such country in the licensed territory, unless sooner terminated.
The License Agreement contemplates that we will seek to work with pharmaceutical companies and clinical trial sites (including comprehensive cancer centers) to initiate clinical trials within timeframes that will meet certain benchmarks. Data from the clinical trials will be the subject of various regulatory filings for marketing approval in applicable countries in the licensed territories. Subject to the receipt of marketing approval, we would be expected to commercialize the licensed products in markets where regulatory approval has been obtained.
| -9- |
Other Significant Agreements and Contracts
Netherlands Cancer Institute
On October 8, 2021, we entered into a Development Collaboration Agreement with the Netherlands Cancer Institute, Amsterdam (“NKI”), one of the world’s leading comprehensive cancer centers, and Oncode Institute, Utrecht, a major independent cancer research center, for a term of three years. The Development Collaboration Agreement was subsequently modified by Amendment No. 1 thereto.
The Development Collaboration Agreement is a preclinical study intended to identify the most promising drugs to be combined with LB-100, and potentially LB-100 analogues, to be used to treat a range of cancers, as well as to identify the specific molecular mechanisms underlying the identified combinations. We agreed to fund the preclinical study, at an approximate cost of 391,000 Euros and provide a sufficient supply of LB-100 to conduct the preclinical study.
On October 3, 2023, we entered into Amendment No. 2 to the Development Collaboration Agreement with NKI, which provides for additional research activities, extends the termination date of the Development Collaboration Agreement by two years to October 8, 2026, and added 500,000 Euros to the operating budget being funded by us.
On October 4, 2024, we entered into Amendment No. 3 to the Development Collaboration Agreement with NKI, which suspended Amendment No. 2 and provided for a new study term of one year and starts upon the dosing of the first patient in the clinical trial at a project cost of 100,000 Euros.
Effective as of June 15, 2022, Dr. René Bernards was appointed to our Board of Directors as an independent director. Dr. Bernards is a leader in the field of molecular carcinogenesis and is employed by NKI.
Intellectual Property
Our intellectual property includes proprietary know-how, proprietary methodologies and extensive clinical validation data and publications. To provide legal protection of our intellectual property, we rely on a combination of patents, licenses, trade secrets, trademarks, confidentiality and non-disclosure clauses and agreements, and other forms of intellectual property protection to define and protect our rights to our products.
Our products are expected to be covered by our patents. These patents now cover sole rights to the composition and synthesis of our LB-100 series of drugs, which is the Company’s lead clinical compound in development. Lixte has filed patent applications covering the treatment of cancer with LB-100. Lixte has also filed joint patent applications with the NIH and the Netherlands Cancer Institute for the treatment of cancer using LB-100 in combination with other drugs like immune checkpoint inhibitors and WEE1 inhibitors (a class of drugs that target and inhibit the WEE1 kinase enzyme that plays a crucial role in regulating cell division).
Patent applications for the LB-100 series (oxabicycloheptanes and oxabicycloheptenes) have been filed in the United States and internationally under the Patent Cooperation Treaty. Patents for composition of matter and for several uses of the LB-100 series have been issued in the United States, Mexico, Australia, Japan, China, Hong Kong, Canada, and by the European Patent Office
The Company strives to protect and enhance the proprietary technology, inventions, and improvements that are commercially important to the development of its business, including seeking, maintaining, and defending its patent rights, which are owned solely by our wholly-owned Delaware subsidiary, Lixte Biotechnology, Inc., except in several instances jointly with one of many of our collaborators. The Company also relies on trade secrets relating to its proprietary pipeline of product candidates and on know-how and continuing technological innovation to develop and strengthen its pipeline. The Company intends to rely on regulatory protection afforded by regulatory agencies through data exclusivity, market exclusivity, and patent term extensions, where available.
The Company’s success will depend in large part on its ability to obtain and maintain patent and other proprietary protection for commercially important technology, inventions and know-how related to its business; defend and enforce its patents; preserve the confidentiality of its trade secrets; and operate without infringing valid and enforceable patents or proprietary rights of third parties. The Company’s ability to stop third parties from making, using, selling, offering to sell, or importing our technology may depend on the extent to which the Company has rights under valid and enforceable licenses, patents, or trade secrets that cover these activities. In some cases, enforcement of these rights may depend on cooperation of the joint owners of our jointly owned patents and patent applications.
| -10- |
With respect to both the Company’s solely and jointly owned intellectual property, the Company cannot be sure that patents will be granted on any of its pending patent applications or on any patent applications filed solely or jointly by the Company in the future; we cannot be sure that any of the Company’s existing patents or any patents that may be granted to us in the future will be commercially useful in protecting the Company’s intended commercial products or therapeutic methods; and the Company cannot be sure that an agency or court would determine that the Company’s solely or jointly owned patents are valid and enforceable.
The patent portfolios for the Company’s most important programs involving the development of the LB-100 series are summarized and presented below, along with related information, as of March 10, 2025, followed by a detailed listing of U.S. and non-U.S. patents that have been issued. The projected patent expiration dates noted below assume that that all required maintenance or annuity fees for the patents are timely paid and that a court or agency does not determine that the patents are invalid or unenforceable.
LB-100. The Company’s lead compound LB-100 is covered by U.S. Patent Nos. 8,822,461 and 7,998,957, which are solely owned by Lixte Biotechnology, Inc. These patents are projected to expire in 2030 or 2028, exclusive of any available patent term extension. Counterpart non-U.S. patents are projected to expire in 2028. Pharmaceutical compositions of LB-100 are covered by U.S. Patent Nos. 10,532,050, 10,023,587 and 8,822,461, which are solely owned by Lixte Biotechnology, Inc. These patents and their non-U.S. counterparts are projected to expire in 2034 or 2028, exclusive of any available patent term extension.
LB-100 Combination Therapy with a Checkpoint Inhibitor. LB-100 combination therapy with a checkpoint inhibitor for treating cancer is covered by U.S. Patent No. 12,168,008 and a pending U.S. patent application, as well as by non-U.S. patents and patent applications. These patents and patent applications are jointly owned by Lixte Biotechnology, Inc., and The United States of America, as represented by the Secretary, Department of Health and Human Services. These patents and patents issuing from these patent applications are projected to expire in 2037, exclusive of any patent term extension.
LB-100 Combination Therapy with Carboplatin, Etoposide and Atezolizumab. LB-100 combination therapy with carboplatin, etoposide and atezolizumab for treating small-cell lung cancer is covered by pending U.S. and non-U.S. patent applications that are solely owned by Lixte Biotechnology, Inc. Patents issuing from these patent applications are projected to expire in 2041, exclusive of any patent term extension.
LB-100 Combination Therapy with Another Investigational Compound. LB-100 combination therapy with one of several other investigational compounds for treating cancer, or preventing, inhibiting or reducing risk of metastasis of cancer, is covered by pending U.S. and non-U.S. patent applications that are jointly owned by Lixte Biotechnology, Inc., and Stichting Het Nederlands Kanker Instituut – Antoni Van Leeuwenhoek Ziekenhuis. Patents issuing from these patent applications are projected to expire in 2043, exclusive of any patent term extension.
LB-100 for Treating Cancer. LB-100 for treating breast cancer, colon cancer, large cell lung cancer, adenocarcinoma of the lung, small cell lung cancer, stomach cancer, liver cancer, ovary adenocarcinoma, pancreas carcinoma, prostate carcinoma, promyelocytic leukemia, chronic myelocytic leukemia or acute lymphocytic leukemia, is covered by U.S. Patent No. 9,079,917, which is solely owned by Lixte Biotechnology, Inc. LB-100 for treating glioblastoma multiforme, medulloblastoma, ovarian cancer, kidney cancer and colorectal cancer is covered by U.S. Patent No. 10,399,993. These patents and their non-U.S. counterparts are projected to expire in 2028, exclusive of any patent term extension.
LB-100 Prodrugs and Analogs. LB-100 prodrugs and analogs are covered by U.S. Patent Nos. 11,866,444, 10,618,908, 10,364,252, 9,988,394, 8,822,461, 8,541,458, 8,426,444, 8,227,473 and 7,998,957, which are solely owned by Lixte Biotechnology, Inc. These patents and their non-U.S. counterparts are projected to expire in 2036, 2030 or 2028, exclusive of any patent term extension. Pharmaceutical compositions of LB-100 prodrugs or analogs are covered by U.S. Patent Nos. 11,931,354, 11,236,102, 10,532,050, 10,023,587, 8,822,461, 8,227,473 and 7,998,957, which are solely owned by Lixte Biotechnology, Inc. These patents and their non-U.S. counterparts are projected to expire in 2034, 2030 or 2028, exclusive of any patent term extension.
| -11- |
Our portfolio of solely or jointly owned U.S. and non-U.S. issued patents is summarized below. We have additional U.S. and non-U.S. patent applications pending.
Oxabicycloheptanes and Oxabicycloheptenes, Their Preparation and Use
| Patent | Issue/Grant Date | Expiration Date | ||
| AU 2008214299 | 1/19/2014 | 2/6/2028 | ||
| CA 2,676,422 | 10/16/2018 | 2/6/2028 | ||
| CN 101662939 | 11/25/2015 | 2/6/2028 | ||
| CN 103788108 | 4/12/2017 | 2/6/2028 | ||
| EP 21245501 | 4/19/2017 | 2/6/2028 | ||
| JP 5693850 | 4/1/2015 | 2/6/2028 | ||
| JP 5666443 | 12/19/2014 | 12/19/2029 | ||
| MX 324705 | 10/21/2014 | 12/19/2029 | ||
| US 7,998,957 | 8/16/2011 | 2/20/2030 | ||
| US 8,227,473 | 7/24/2012 | 3/11/2030 | ||
| US 8,426,444 | 4/23/2013 | 2/6/2028 | ||
| US 8,541,458 | 9/24/2013 | 7/17/2029 | ||
| US 8,822,461 | 9/2/2014 | 2/6/2028 | ||
| US 9,079,917 | 7/14/2015 | 2/6/2028 | ||
| US 10,023,587 | 7/17/2018 | 2/6/2028 | ||
| US 10,399,993 | 9/3/2019 | 2/6/2028 |
1EP 2124550 validated and pending in Germany, Spain, France, United Kingdom and Italy
Formulations of Oxabicycloheptanes and Oxabicycloheptenes
| Patent | Issue/Grant Date | Expiration Date | ||
| AU 2014251087 | 5/2/2019 | 4/8/2034 | ||
| CA 2909160 | 5/25/2021 | 4/8/2034 | ||
| CN 105209036 | 10/26/2018 | 4/8/2034 | ||
| EP 29836611 | 5/29/2024 | 4/8/2034 | ||
| IL 241945 | 4/30/2019 | 4/8/2034 | ||
| US 10,532,050 | 1/14/2020 | 7/5/2034 | ||
| US 11,931,354 | 3/19/2024 | 4/8/2034 |
1EP 2983661 validated and pending as Unitary Patent, and in Spain, United Kingdom and Switzerland
Process of Synthesizing 3-(4-Methylpiperazine-1-Carbonyl)-7-Oxabicyclo [2.2.1] Heptane-2-Carboxylic Acid
| Patent | Issue/Grant Date | Expiration Date | ||
| US 9,994,584 | 6/12/2018 | 10/14/2035 |
| -12- |
Oxabicycloheptane Prodrugs
| Patent | Issue/Grant Date | Expiration Date | ||
| AU 2016263079 | 8/15/2019 | 5/12/2036 | ||
| EP 32942871 | 4/8/2020 | 5/12/2036 | ||
| EP 37362752 | 7/3/2024 | 5/12/2036 | ||
| HK 1247576 | 3/5/2021 | 5/12/2036 | ||
| IL 255516 | 2/27/2020 | 5/12/2036 | ||
| IL 272027 | 22/1/2022 | 5/12/2036 | ||
| IN 394963 | 4/19/2022 | 5/12/2036 | ||
| JP 7187023 | 12/2/2022 | 5/12/2036 | ||
| MX 386975 | 10/12/2021 | 5/12/2036 | ||
| MX 393461 | 6/28/2022 | 5/12/2036 | ||
| TW I693226 | 5/11/2020 | 5/12/2036 | ||
| TW I757720 | 3/11/2022 | 5/12/2036 | ||
| US 9,988,394 | 6/5/2018 | 5/13/2036 | ||
| US 10,364,252 | 7/30/2019 | 5/13/2036 | ||
| US 10,618,908 | 4/14/2020 | 5/13/2036 | ||
| US 11,236,102 | 2/1/2022 | 5/13/2036 | ||
| US 11,866,444 | 1/9/2024 | 5/13/2036 |
1EP 3294287 validated and pending in Austria, Switzerland, Czechia, Germany, Denmark, Spain, France, United Kingdom, Hungary, Ireland, Italy, Netherlands, and Sweden
2EP 3736275 validated and pending as Unitary Patent, and in Spain, United Kingdom and Switzerland
Oxabicycloheptanes for Modulation of Immune Response
| Patent | Issue/Grant Date | Expiration Date | ||
| AU 2017370731 | 9/15/2022 | 12/8/2037 | ||
| CN 110234647 | 5/23/2023 | 12/8/2037 | ||
| EP 35516291 | 11/15/2023 | 12/8/2037 | ||
| HK 40015901 | 4/12/2024 | 12/8/2037 | ||
| IL 267134 | 7/2/2022 | 12/8/2037 | ||
| IL 290857 | 2/2/2023 | 12/8/2037 | ||
| JP 7246309 | 3/16/2023 | 12/8/2037 | ||
| MX 396386 | 10/12/2022 | 12/8/2037 | ||
| US 12,168,008 | 12/17/2024 | 12/8/2037 |
1EP 3551629 validated and pending in Belgium, Germany, Denmark, Spain, France, United Kingdom, Ireland, Iceland, Italy, Netherlands, Norway, Sweden and Switzerland
The Market
Anti-Cancer Drugs
We believe that the mechanism by which compounds of the LB-100 series affects cancer cell growth is different from cancer agents currently approved for clinical use. Lead compounds of the LB-100 series have activity against a broad spectrum of common and rarer human cancers in cell culture systems. In addition, lead compounds of the LB-100 series have anti-cancer activity in animal models of glioblastoma multiforme, neuroblastoma, and medulloblastoma, all cancers of neural tissue. Lead compounds of the LB-100 series also have activity against melanoma, breast cancer and sarcoma in animal models and enhance the effectiveness of commonly used anti-cancer drugs in animal models. The enhancement of anti-cancer activity of these commonly-used anti-cancer drugs occurs at doses of LB-100 that do not significantly increase toxicity in animals. It is therefore hoped that when combined with standard anti-cancer regimens against many tumor types, LB-100 will improve therapeutic benefit without unacceptable toxicity in humans.
LB-100 is part of a pioneering effort in an entirely new field of cancer biology – activation lethality – that is advancing a new treatment paradigm. The Company is the only company that has a drug in clinical trials with demonstrated capacity to over-activate oncogenic signaling. The pre-clinical data obtained with LB-100 were recently posted online in a paper titled “Paradoxical Activation of Oncogenic Signaling as a Cancer Treatment Strategy” in the scientific journal Cancer Discovery, and were published in the July 2024 issue of Cancer Discovery. This study showed that LB-100 triggers hyper-activation of the signals that are responsible for the deregulated proliferation of cancer cells, thus leading to cell death. This approach is the opposite of most of the current generation of cancer therapies and opens potentially new treatment strategies.
| -13- |
Marketing Plan
Our primary goal to date has been to take our primary compound, LB-100, through Phase 2 clinical trials evaluating whether LB-100 will enhance anti-cancer therapies. Because of the novelty and spectrum of activity of LB-100, we believe it is reasonably likely we may find a partner in the pharmaceutical industry with interest in this compound at some stage of its clinical development. However, we would prefer to delay the partnering/licensing decision until the potential value of our products are augmented by demonstrating there is no impediment to clinical evaluation and a therapeutic dose level is determined in clinical trials. Demonstration of clinical usefulness would be expected to substantially increase the value of our product.
Product Development
We are subject to FDA regulations as it conducts clinical trials. Additionally, any product for which we obtain marketing approval, along with the manufacturing processes, post-approval clinical data and promotional activities for such product, will be subject to continual review and periodic inspections by the FDA and other regulatory bodies. Even if regulatory approval of a product is granted, the approval may be subject to limitations on the indicated uses for which the product may be marketed or contain requirements for costly post-marketing testing and surveillance to monitor the safety or efficacy of the product. Later discovery of previously unknown problems with our products, including unanticipated adverse events or adverse events of unanticipated severity or frequency, manufacturer or manufacturing processes, or failure to comply with regulatory requirements, may result in restrictions on such products or manufacturing processes, withdrawal of the products from the market, voluntary or mandatory recall, fines, suspension of regulatory approvals, product seizures, injunctions or the imposition of civil or criminal penalties.
Competition
The life sciences industry is highly competitive and subject to rapid and profound technological change. Our present and potential competitors include major pharmaceutical companies, as well as specialized biotechnology and life sciences firms in the United States and in other countries. Most of these companies have considerably greater financial, technical and marketing resources than we do. Additionally, mergers and acquisitions in the pharmaceutical and biotechnology industries may result in even more resources being concentrated in our competitors. Our existing or prospective competitors may develop processes or products that are more effective than ours or be more effective at implementing their technologies to develop commercial products faster. Our competitors may succeed in obtaining patent protection and/or receiving regulatory approval for commercializing products before we do. Developments by our competitors may render our product candidates obsolete or non-competitive.
We also experience competition from universities and other research institutions, and we are likely to compete with others in acquiring technology from those sources. There can be no assurance that other organizations will not develop technologies with significant advantages over those that we are seeking to develop. Any such development could harm our business.
We compete with universities and other research institutions engaged in research in these areas. Many of our competitors have greater technical and financial resources than we do.
Our ability to compete successfully is based on numerous factors, including:
| ● | the cost-effectiveness of any product that we ultimately commercialize relative to competing products; | |
| ● | the ease of use and ready availability of any product that we bring to market; and | |
| ● | the relative speed with which we are able to bring any product resulting from its research to market in our target markets. |
If we are unable to distinguish our products from competing products, or if competing products reach the market first, we may be unable to compete successfully with current or future competitors.
| -14- |
Employees and Human Capital Resources
As of March 14, 2025, we had two officer/employees, our Chief Executive Officer and our Chief Financial Officer, and one consultant, our Chief Medical Officer. The Company relies to a significant extent on outside consultants and advisors with various technical skills and expertise that the Company can draw on as necessary to conduct its research and development and clinical trial programs. We consider our relationship with our employees to be good. Our future performance depends significantly upon the continued service of our key personnel and our ability to attract highly skilled employees. We provide our officer/employees and consultants with opportunities for equity ownership.
Facilities
As of March 14, 2025, we do not operate or lease any facilities. We contract out research and development activities, drug production, and drug storage to various commercial laboratories, drug manufacturers and storage facilities.
Government Regulation
Our business is subject to the regulations of the FDA as it conducts clinical trials. Clinical trials are research studies to answer specific questions about new therapies or new ways of using known treatments. Clinical trials determine whether new drugs or treatments are both safe and effective and the FDA has determined that carefully conducted clinical trials are the fastest and safest way to find treatments that work in people.
The FDA also requires that an independent review body consider the benefits and risks of a clinical trial and grant approval for the proposed study including selecting of initial doses, plans for escalation of dose, plans for modification of dose if toxicity is encountered, plans for monitoring the wellbeing of individuals participating in the study, and for defining and measuring, to the extent possible, any untoward effects related to drug administration. Serious adverse effects, such as life-threatening toxicities and death, are immediately reportable to the review body and to the FDA. To minimize risk when studying a new drug, the initial dose is well below that expected to cause any toxicity. No more than three patients are entered at a given dose. In general, a dose is not escalated within an individual patient. Once safety is established by the absence of toxicity or low toxicity in a group of three patients, a planned higher dose is then evaluated in a subsequent group of three individuals and so on until dose-limiting toxicity is encountered. The dose level producing acceptable toxicity is then selected as the dose level to be evaluated in Phase 2 trials. Thus, the goal of Phase 1 studies is to determine the appropriate dose level for evaluation of drug efficacy in patients with cancer.
In addition to regulations imposed by the FDA, depending on our future activities, we may become subject to regulation under various federal and state statutes and regulations, such as the Occupational Safety and Health Act, the Environmental Protection Act, the Toxic Substances Control Act, the Research Conservation and Recovery Act, national restrictions on technology transfer, and import, export and customs regulations. From time to time, other federal agencies and congressional committees have indicated an interest in implementing further regulation of biotechnology applications. We are not able to predict whether any such regulations will be adopted or whether, if adopted, such regulations will apply to our business, or whether we or our collaborators would be able to comply with any applicable regulations.
In addition, as we intend to market our products in international markets, we will be required to obtain separate regulatory approvals from the European Union and many other foreign jurisdictions. Approval by the FDA does not ensure approval by regulatory authorities in other countries, and approval by one foreign regulatory authority does not ensure approval by regulatory authorities in other foreign countries or by the FDA. We may not be able to file for regulatory approvals and may not receive necessary approvals to commercialize our products in any market.
| -15- |
Legal Proceedings
The Company may be subject to legal claims and actions from time to time as part of its business activities. We are not currently subject to any threatened or pending lawsuits, legal claims or legal proceedings.
ITEM 1A. RISK FACTORS
The following risk factors, together with the other information presented in this document, including the financial statements and the notes thereto, should be considered by investors.
Risks Related to Our Financial Resources and Capital Needs
We are engaged in early-stage research and as such might not be successful in our efforts to develop a portfolio of commercially viable products.
A key element of our strategy is to develop LB-100 in combination with other anti-cancer therapies to treat cancer. We are seeking to do so through our internal research programs or strategic partnerships. A significant portion of the research and development that we are conducting involves new and unproven technologies. Research programs to identify new disease targets and product candidates or to develop them require substantial technical, financial and human resources whether or not any candidates or technologies are ultimately identified or proven successful. Our research programs might initially show promise in identifying potential product candidates, yet fail to yield product candidates for clinical development for the following reasons:
| ● | the research methodology used might not be successful in identifying potential product candidates; or | |
| ● | product candidates for drugs might on further study be shown to have harmful side effects or other characteristics that indicate they are unlikely to be effective drugs. |
If we are unable to discover suitable potential product candidates, develop additional delivery technologies through internal research programs or strategic partnerships, or in-license suitable products or delivery technologies on acceptable business terms, our business prospects will suffer. Even if we discover additional product candidates, new clinical trials of one or more additional drug candidates may show that these product candidates are unsafe or ineffective.
We have incurred substantial losses since our inception and anticipate that we will continue to incur substantial and increasing losses for the foreseeable future.
We are a clinical-stage biopharmaceutical company that uses biomarker technology to identify enzyme targets associated with serious common diseases and then design novel compounds to attack those threats. We do not have any products approved by a regulatory authority and have not generated any revenue from collaboration or licensing agreements or product sales to date, and have incurred significant research, development and other expenses related to our ongoing operations and expect to continue to incur such expenses. As a result, we have not been profitable and have incurred significant operating losses since our inception. For the years ended December 31, 2024 and 2023, we reported a net loss of $3,585,965 and $5,087,029, respectively. As of December 31, 2024 and 2023, we had an accumulated deficit of $52,067,693 and $48,481,728, respectively.
We do not expect to generate revenues for many years, if at all. We expect to continue to incur significant expenses and operating losses for the foreseeable future. We anticipate these losses to increase as we continue to research, develop and seek regulatory approvals for one or more of our product candidates and any additional product candidates we might acquire, and potentially begin to commercialize product candidates that might achieve regulatory approval. We might also encounter unforeseen expenses, difficulties, complications, delays and other unknown factors that could adversely affect our business. The size of our future net losses will depend, in part, on the rate of future growth of our expenses and our ability to generate revenues. Our expenses will further increase as we:
| ● | conduct clinical trials of our lead product candidate, LB-100; | |
| ● | in-license or acquire rights to, and pursue development of, other products, product candidates or technologies; |
| -16- |
| ● | hire additional clinical, administrative, manufacturing, quality control, quality assurance and scientific personnel; | |
| ● | seek marketing approval for any product candidates that successfully complete clinical trials; | |
| ● | develop our outsourced manufacturing and commercial activities and establish sales, marketing and distribution capabilities, if we receive, or expect to receive, marketing approval for any product candidates; | |
| ● | maintain, expand and protect our intellectual property portfolio; and | |
| ● | add operational, financial and management information systems and personnel. |
Our independent registered public accounting firm has expressed substantial doubt about our ability to continue as a going concern.
The Company’s consolidated financial statements have been presented on the basis that it will continue as a going concern, which contemplates the realization of assets and satisfaction of liabilities in the normal course of business. The Company has no recurring source of revenue and has experienced negative operating cash flows since inception, and management has determined that substantial doubt exists about the Company’s ability to continue as a going concern. As a result, our independent registered public accounting firm has included an explanatory paragraph in their report with respect to this uncertainty that accompanies our audited consolidated financial statements as of and for the year ended December 31, 2024. This going concern opinion could materially limit our ability to raise additional funds through the sale of equity securities in the future, and subsequent reports by our independent registered public accounting firm on our consolidated financial statements may also include an explanatory paragraph with respect to our ability to continue as a going concern.
We need significant additional financing to fund our operations and complete the development and, if approved, the commercialization of our lead product candidate, LB-100. If we are unable to raise capital when needed, we could be forced to delay, reduce or eliminate our product development programs or commercialization efforts.
We expect that our existing cash resources as of December 31, 2024 will provide sufficient working capital resources to fund our operations, including our clinical trial programs with respect to the development of our lead anti-cancer clinical compound LB-100, through approximately September 30, 2025. Our existing cash resources will not be sufficient to complete development of and obtain regulatory approval for our lead product candidate, and we will need to raise significant additional capital to be able to continue our efforts in this regard. The Company estimates that it will need to raise additional capital to fund its operations by mid-2025, including its various clinical trial commitments, to be able to proactively manage its current business plan during the remainder of 2025 and during 2026. In addition, our operating plan might change as a result of many factors currently unknown to us, including possible additional clinical trials, and we might need additional funds sooner than planned. The Company is considering various strategies and alternatives to obtain the required additional capital.
We expect to expend substantial resources for the foreseeable future to continue the clinical development and production of our lead product candidate. These expenditures will include costs associated with research and development, potentially acquiring new product candidates or technologies, conducting preclinical studies and clinical trials and potentially obtaining regulatory approvals and manufacturing products.
Budgets and future capital requirements depend on many factors, including:
| ● | the scope, progress, results and costs of our ongoing and planned development programs for our lead product candidate, as well as any additional clinical trials we undertake to obtain data sufficient to seek marketing approval for our lead product candidate; |
|
| ● | the timing of, and the costs involved in, obtaining regulatory approvals for our lead drug candidate if our clinical trials are successful; | |
| ● | the cost of commercialization activities for our lead product candidate, if it is approved for sale, including marketing, sales and distribution costs; |
| -17- |
| ● | the cost of manufacturing our lead product candidate for clinical trials in preparation for regulatory approval, including the cost and timing of process development, manufacturing scale-up and validation activities; |
|
| ● | our ability to establish and maintain strategic licensing or other arrangements and the financial terms of such agreements; | |
| ● | the costs to in-license future product candidates or technologies; | |
| ● | the costs involved in preparing, filing, prosecuting, maintaining, expanding, defending and enforcing patent claims, including litigation costs and the outcome of such litigation; | |
| ● | the costs in defending and resolving future derivative and securities class action litigation; | |
| ● | our operating expenses; and | |
| ● | the emergence of competing technologies or other adverse market developments. |
Additional funds might not be available when we need them on terms that are acceptable to us, or at all. We have no committed source of additional capital. If adequate funds are not available to us on a timely basis, we might not be able to continue as a going concern or we might be required to delay, limit, reduce or terminate preclinical studies, clinical trials or other development activities for our product candidates or target indications, or delay, limit, reduce or terminate our establishment of sales and marketing capabilities or other activities that may be necessary to commercialize our lead product candidate.
We currently have no source of revenues. We might never generate revenues or achieve profitability.
Currently, we do not generate any revenues from product sales or otherwise. Even if we are able to successfully achieve regulatory approval for our lead product candidate, we do not know when we will generate revenues or become profitable, if at all. Our ability to generate revenues from product sales and achieve profitability will depend on our ability to successfully commercialize products, including our lead product candidate, LB-100, and any other product candidates that we might develop, in-license or acquire in the future. Our ability to generate revenues and achieve profitability also depends on a number of additional factors, including our ability to:
| ● | successfully complete development activities, including the necessary clinical trials; | |
| ● | complete and submit a New Drug Application (“NDA”) to the FDA and obtain U.S. regulatory approval for an indication for which there is a commercial market; | |
| ● | complete and submit applications to foreign regulatory authorities; | |
| ● | obtain regulatory approval in territories with viable market sizes; | |
| ● | obtain coverage and adequate reimbursement from third parties, including government and private payors; | |
| ● | set commercially viable prices for our intended product, if any; | |
| ● | establish and maintain supply and manufacturing relationships with reliable third parties and/or build our own manufacturing facility and ensure adequate, legally and globally compliant manufacturing of bulk drug substances and drug products to maintain that supply; | |
| ● | develop distribution processes for our lead product candidate; | |
| ● | develop commercial quantities of our lead product candidate, once approved, at acceptable cost levels; | |
| ● | obtain additional funding, if required to develop and commercialize our lead product candidate; |
| -18- |
| ● | develop a commercial organization capable of sales, marketing and distribution for any products we intend to sell ourselves, in the markets in which we choose to commercialize on our own; | |
| ● | achieve market acceptance of one or more of our intended products; | |
| ● | attract, hire and retain qualified personnel; and | |
| ● | protect our rights in our intellectual property portfolio. |
Our revenues for any product candidate for which regulatory approval is obtained will be dependent, in part, upon the size of the markets in the territories for which it gains regulatory approval, the accepted price for the product, the ability to get reimbursement at any price, and whether we own the commercial rights for that territory. If the number of our addressable-disease patients is not as significant as our estimates, the indication approved by regulatory authorities is narrower than we expect, or the reasonably accepted population for treatment is narrowed by competition, physician choice or treatment guidelines, we might not generate significant revenues from sales of such products, even if approved. In addition, we anticipate incurring significant costs associated with commercializing any approved product candidate. As a result, even if we generate revenues, we might not become profitable and might need to obtain additional funding to continue operations. If we fail to become profitable or are unable to sustain profitability on a continuing basis, then we might be unable to continue our operations at planned levels and might be forced to reduce our operations.
Our ability to use net operating losses to offset future taxable income might be subject to limitations.
At December 31, 2024, the Company has available net operating loss carryforwards for federal and state income tax purposes of approximately $31,067,000 and $35,836,000, respectively. Federal net operating losses from tax years preceding 2018, if not utilized earlier, expire through 2038. Federal net operating losses generated in a tax year beginning after 2017 have an indefinite carryforward period. The utilization of federal net operating loss carryforwards is subject to various limitations.
The state net operating loss carryovers include approximately $19,141,000 that were incurred in the State of New York and approximately $16,695,000 that were incurred in the State of California, which are subject to various restrictions and limitations.
In addition, under Section 382 of the Internal Revenue Code of 1986, as amended, and certain corresponding provisions of state law, if a corporation undergoes an “ownership change”, which is generally defined as a greater than 50% change, by value, in the ownership of its equity over a three-year period, the corporation’s ability to use its pre-change NOL carryforwards and other pre-change tax attributes to offset its post-change income might be limited.
Risks Related to the Development and Regulatory Approval of Our Product Candidates
Clinical-stage biopharmaceutical companies with product candidates in clinical development face a wide range of challenging activities which might entail substantial risk.
We are a clinical-stage biopharmaceutical company with a lead product candidate in clinical development. The success of our lead product candidate will depend on several factors, including the following:
| ● | designing, conducting and successfully completing preclinical development activities, including preclinical efficacy and IND-enabling studies, for our lead product candidate or product candidates that we might, in the future, in-license or acquire; | |
| ● | designing, conducting and completing clinical trials with positive results for our lead product candidate; | |
| ● |
receipt of regulatory approvals from applicable authorities;
|
|
| ● | obtaining and maintaining patent and trade secret protection and regulatory exclusivity for our lead product candidate; |
| -19- |
| ● | making arrangements with third party manufacturers, receiving regulatory approval of our manufacturing processes and our third party manufacturers’ facilities from applicable regulatory authorities and ensuring adequate supply of drug product; | |
| ● | manufacturing our lead product candidate at an acceptable cost; | |
| ● | effectively launching commercial sales of our lead product candidate, if approved, whether alone or in collaboration with others; | |
| ● | achieving acceptance of our lead product candidate, if approved, by patients, the medical community and third party payors; | |
| ● | effectively competing with other therapies; | |
| ● | if our lead product candidate is approved, obtaining and maintaining coverage and adequate reimbursement by third party payors, including government payors, for our lead product candidate; | |
| ● | complying with all applicable regulatory requirements, including FDA current Good Clinical Practices (“GCP”), Current Good Manufacturing Practices (“CGMP”), and standards, rules and regulations governing promotional and other marketing activities; | |
| ● | maintaining a continued acceptable safety profile of the lead product candidate during development and following approval. |
If we do not achieve one or more of these factors in a timely manner or at all, we could experience significant delays or an inability to successfully develop and commercialize our lead product candidate, which could materially harm our business.
We might find it difficult to enroll patients in our clinical trials which could delay or prevent the start of clinical trials for our product candidate.
Identifying and qualifying patients to participate in clinical trials of our lead product candidate is essential to our success. The timing of our clinical trials depends in part on the rate at which we can recruit patients to participate in clinical trials of our lead product candidate, and we might experience delays in our clinical trials if we encounter difficulties in enrollment. If we experience delays in our clinical trials, the timeline for obtaining regulatory approval of our lead product candidate will most likely be delayed.
Many factors might affect our ability to identify, enroll and maintain qualified patients, including the following:
| ● | eligibility criteria of our ongoing and planned clinical trials with specific characteristics appropriate for inclusion in our clinical trials; | |
| ● | design of the clinical trial; | |
| ● | size and nature of the patient population; | |
| ● | patients’ perceptions as to risks and benefits of the lead product candidate under study and the participation in a clinical trial generally in relation to other available therapies, including any new drugs that might be approved for the indications we are investigating; | |
| ● | the availability and efficacy of competing therapies and clinical trials; | |
| ● | pendency of other trials underway in the same patient population; | |
| ● | willingness of physicians to participate in our planned clinical trials; |
| -20- |
| ● | severity of the disease under investigation; | |
| ● | proximity of patients to clinical sites; | |
| ● | patients who are noncompliant or do not otherwise complete the trials; and | |
| ● | issues with a contract research organization (a “CRO”) and/or with other vendors that are involved with our clinical trials. |
We might not be able to initiate or continue to support clinical trials of LB-100, our lead product candidate, for one or more indications, or any future product candidates if we are unable to locate and enroll a sufficient number of eligible participants in these trials as required by the FDA or one or more other regulatory authorities. Even if we are able to enroll a sufficient number of patients in our clinical trials, if the pace of enrollment is slower than we expect, the development costs for our lead product candidate might increase and the completion of our trials might be delayed or our trials could become too expensive to complete.
If we experience delays in the completion of, or termination of, any clinical trials of our lead product candidate, the commercial prospects of our lead product candidate could be harmed, and our ability to generate product revenue from any of our product candidates could be delayed or prevented. In addition, any delays in completing our clinical trials would likely increase our overall costs, impair product candidate development and jeopardize our ability to obtain regulatory approval relative to our current plans. Any of these occurrences might harm our business, financial condition, and prospects significantly.
The results of preclinical studies or earlier clinical trials are not necessarily predictive of future results. Our lead product candidate in clinical trials, and any other product candidates that might advance into clinical trials, might not have favorable results in later clinical trials or receive regulatory approval.
Success in preclinical studies and early clinical trials does not ensure that later clinical trials will generate adequate data to demonstrate the efficacy and safety of an investigational drug. A number of companies in the pharmaceutical and biotechnology industries, including those with greater resources and experience than we have, have suffered significant setbacks in clinical trials, even after seeing promising results in earlier preclinical studies or clinical trials.
Despite the results reported in earlier preclinical studies or clinical trials for our lead product candidate, we do not know whether the clinical trials that we might conduct will demonstrate adequate efficacy and safety to result in regulatory approval to market our lead product candidate for a particular indication, in any particular jurisdiction. Efficacy data from prospectively designed trials might differ significantly from those obtained from retrospective subgroup analyses. If later-stage clinical trials do not produce favorable results, our ability to achieve regulatory approval for our lead product candidate might be adversely impacted. Even if we believe that we have adequate data to support an application for regulatory approval to market our lead product candidate or any future product candidates, the FDA or other regulatory authorities might not agree and might require that we conduct additional clinical trials.
Clinical drug development involves a lengthy and expensive process with an uncertain outcome.
Clinical testing is expensive and can take many years to complete, with the outcome inherently uncertain. Failure can occur at any time during the clinical trial process. Before obtaining approval from regulatory authorities for the sale of our lead product candidate, we must conduct extensive clinical trials to demonstrate the safety and efficacy of our lead product candidate in humans. Prior to initiating clinical trials, a sponsor must complete extensive preclinical testing of a product candidate, including, in most cases, preclinical efficacy experiments as well as IND-enabling toxicology studies. These experiments and studies might be time-consuming and expensive to complete. The necessary preclinical testing might not be completed successfully for a preclinical product candidate and a potentially promising product candidate might therefore never be tested in humans. Once it commences, clinical testing is expensive, difficult to design and implement, can take many years to complete and is uncertain as to outcome. A failure of one or more clinical trials can occur at any stage of testing. The outcome of preclinical testing and early clinical trials might not be predictive of the success of later clinical trials, and interim results of a clinical trial do not necessarily predict final results. Moreover, preclinical and clinical data are often susceptible to varying interpretations and analyses, and many companies that have believed their product candidates performed satisfactorily in preclinical studies and clinical trials have nonetheless failed to obtain marketing approval of their products. We might experience numerous unforeseen events during drug development that could delay or prevent our ability to receive marketing approval or commercialize our lead product candidate. In particular, clinical trials of our lead product candidate might produce inconclusive or negative results. We have limited data regarding the safety, tolerability and efficacy of our lead product candidate. Clinical trials also require the review and oversight of an institutional review board (“IRB”). An inability or delay in obtaining IRB approval could prevent or delay the initiation and completion of clinical trials, and the FDA might decide not to consider any data or information derived from a clinical investigation not subject to initial and continuing IRB review and approval.
| -21- |
We might experience delays in our ongoing or future clinical trials, and we do not know whether planned clinical trials will begin or enroll subjects on time, will need to be redesigned or will be completed on schedule, if at all. There can be no assurance that the FDA or another regulatory agency will not put clinical trials of our lead product candidate on hold in the future. Clinical trials might be delayed, suspended or prematurely terminated for a variety of reasons, such as:
| ● | delay or failure in reaching agreement with the FDA or a foreign regulatory authority on a clinical trial design that we are able to execute; | |
| ● | delay or failure in obtaining authorization to commence a trial or inability to comply with conditions imposed by a regulatory authority regarding the scope or design of a trial; | |
| ● | delay or failure in reaching agreement on acceptable terms with prospective CROs and clinical trial sites, the terms of which can be subject to extensive negotiation and may vary significantly among different CROs and trial sites; | |
| ● | delay or failure in obtaining IRB approval or the approval of other reviewing entities, including comparable foreign regulatory authorities, to conduct a clinical trial at each site; | |
| ● | withdrawal of clinical trial sites from our clinical trials or the ineligibility of a site to participate in our clinical trials; | |
| ● | delay or failure in recruiting and enrolling suitable subjects to participate in a trial; | |
| ● | delay or failure in subjects completing a trial or returning for post-treatment follow-up; | |
| ● | clinical sites and investigators deviating from trial protocol, failing to conduct the trial in accordance with regulatory requirements, or dropping out of a trial; | |
| ● | inability to identify and maintain a sufficient number of trial sites, many of which may already be engaged in other clinical trial programs, including some that may be for the same indication; | |
| ● | failure of our third party clinical trial managers, CROs, clinical trial sites, contracted laboratories or other third party vendors to satisfy their contractual duties, meet expected deadlines or return trustworthy data; | |
| ● | delay or failure in adding new trial sites; | |
| ● | interim results or data that are ambiguous or negative or are inconsistent with earlier results or data; | |
| ● | alteration of trial design necessitated by re-evaluation of design assumptions based upon observed data; | |
| ● | feedback from the FDA, the IRB or a foreign regulatory authority, or results from earlier stage or concurrent preclinical studies and clinical trials, that might require modification to the protocol for a trial; | |
| ● | a decision by the FDA, the IRB, a foreign regulatory authority, or us to suspend or terminate clinical trials at any time for safety issues or for any other reason; | |
| ● | unacceptable risk-benefit profile, unforeseen safety issues or adverse side effects; |
| -22- |
| ● | failure to demonstrate a benefit from using a product candidate; | |
| ● | difficulties in manufacturing, obtaining, from one or more third parties, or qualifying sufficient quantities of a product candidate to start or to use in clinical trials; | |
| ● | lack of adequate funding to continue a trial, including the incurrence of unforeseen costs due to enrollment delays, requirements to conduct additional studies or increased expenses associated with the services of our CROs and other third parties; or | |
| ● | changes in governmental regulations or administrative actions or lack of adequate funding to continue a clinical trial. |
If we experience delays in the completion or termination of any clinical trial of our lead product candidate, the approval and commercial prospects of our lead product candidate will be harmed, delaying our ability to generate product revenues from such product candidate and our costs will most likely increase. The required regulatory approvals may also be delayed, thereby jeopardizing our ability to commence product sales and generate revenues and the period of commercial exclusivity for our intended product may be shortened. Regulatory approval of our lead product candidate may be denied for the same reasons that caused the delay.
Risks associated with operating in foreign countries could materially adversely affect our product development.
We are currently conducting clinical trials in Spain and the Netherlands. Consequently, we will also be subject to risks related to operating in foreign countries. Risks associated with conducting operations in foreign countries include:
| ● | differing regulatory requirements for drug approvals and regulation of approved drugs in foreign countries; | |
| ● | more stringent privacy requirements for data to be supplied to our operations in the United States, but generated outside of the United States, e.g., General Data Protection Regulation in the European Union; | |
| ● | unexpected changes in tariffs, trade barriers and regulatory requirements; | |
| ● | economic weakness, including inflation, or political instability in particular foreign countries, economies or markets; | |
| ● | compliance with tax, employment, immigration and labor laws for employees living or traveling abroad; | |
| ● | foreign taxes, including withholding or payroll taxes; |
| ● | differing payor reimbursement regimes, governmental payors or patient self-pay systems and price controls; | |
| ● | foreign currency fluctuations, which could result in increased operating expenses or reduced revenues, and other obligations incident to doing business or operating in another country; | |
| ● | workforce uncertainty in countries where labor unrest is more common than in the United States; | |
| ● | production shortages resulting from any events affecting raw material supply or manufacturing capabilities abroad; and | |
| ● | business interruptions resulting from geopolitical actions or events, including civil or political unrest (such as the ongoing conflict between Ukraine and Russia), sanctions, war and terrorism. |
| -23- |
Our current and future product candidates, the methods used to deliver them or their dosage levels may cause undesirable side effects or have other properties that could delay or prevent their regulatory approval, limit the commercial profile of an approved label or result in significant negative consequences following any regulatory approval.
Undesirable side effects caused by our current or future product candidates, their delivery methods or dosage levels could cause us, our collaborators or regulatory authorities to interrupt, delay or halt clinical trials and could result in a more restrictive label or the delay or denial of regulatory approval or termination of clinical trials by the FDA or other foreign regulatory authorities; or an IRB, that approves and, monitors biomedical research to protect the rights and welfare of human subjects. As a result of safety or toxicity issues that we might experience in our clinical trials, or negative or inconclusive results from the clinical trials of others for drug candidates that might be similar to our own, we might not receive approval to market our current lead product candidate or any product candidates we may pursue, which could prevent us from ever generating revenues or achieving profitability. Results of our trials could reveal an unacceptably high severity or incidence of side effects. In such an event, our trials or those or our collaborators could be suspended or terminated, and the FDA or foreign regulatory authorities could order us or our collaborators to cease further development of or deny approval of our current or any future product candidates for any or all targeted indications. Any drug-related side effects could also affect patient recruitment or the ability of enrolled subjects to complete clinical trials or result in potential product liability claims. Any of these occurrences could have a material adverse effect on our business, results of operations, financial condition, cash flows and future prospects.
Additionally, if our lead product candidate receives regulatory approval, and we or others later identify undesirable side effects caused by such product, a number of potentially significant negative consequences could result, including that:
| ● | we may be forced to suspend marketing of such product; | |
| ● | regulatory authorities might withdraw their approvals of such product; | |
| ● | regulatory authorities might require additional warnings on the label that could diminish the usage or otherwise limit the commercial success of such product; | |
| ● | we may be required to conduct post-marketing studies; | |
| ● | we may be required to change the way the product is administered; | |
| ● | we could be sued and held liable for harm caused to subjects or patients; and | |
| ● | our reputation may suffer. |
Any of these events could prevent us from achieving or maintaining market acceptance of our lead product candidate, if approved.
Our product development program might not uncover all possible adverse events that patients who take our lead product candidate may experience. The number of subjects exposed to our lead product candidate and the average exposure time in the clinical development program might be inadequate to detect rare adverse events or chance findings that might only be detected once the product is administered to more patients and for greater periods of time.
Clinical trials by their nature utilize a sample of the potential patient population. However, with a limited number of subjects and limited duration of exposure, we cannot be fully assured that rare and severe side effects of our lead product candidate will be uncovered. Such rare and severe side effects might only be uncovered with a significantly larger number of patients exposed to our lead product candidate. If such safety problems occur or are identified after our lead product candidate reaches the market, the FDA might require that we amend the labeling of the product or recall the product, or might even withdraw approval for the product.
There is a risk that one or more of our clinical trials could be placed on hold by regulatory authorities due to serious adverse events (SAEs) related to our drug candidate or to another company’s drug used in combination in one of our clinical trials.
It is possible that the SAEs could be attributable to our drug candidate and could include, but not be limited to, unexpected severe side effects, treatment-related deaths, or long-term health complications. A dose given could result in non-tolerable adverse events defined as dose-limiting toxicity (DLT). When two DLTs occur at the same dose-level that dose-level is considered too high and unsafe. Further treatment is only allowed at lower dose-levels that have previously been found safe.
| -24- |
The principal investigator of the colorectal study testing LB-100 in combination with atezolizumab (Roche PD-L1 inhibitor) is currently investigating two SAEs observed in the clinical trial that was launched in August 2024. The Institutional Review Board of the Netherlands Cancer Institute has put the colorectal cancer study on hold, as more fully discussed at “Item 7. Management’s Discussion and Analysis of Financial Condition and Results of Operations – Specific Risks Associated with the Company’s Business Activities – Serious Adverse Events”.
Our future success is dependent on the regulatory approval of our lead product candidate.
Our business is dependent on our ability to obtain regulatory approval for our lead product candidate in a timely manner. We cannot commercialize our lead product candidate in the United States without first obtaining regulatory approval for the product from the FDA. Similarly, we cannot commercialize our lead product candidate outside of the United States without obtaining regulatory approval from one or more foreign regulatory authorities. Before obtaining regulatory approvals for the commercial sale of our lead product candidate for a target indication, we must demonstrate with substantial evidence gathered in preclinical studies and clinical trials, that the product candidate is safe and effective for use for that target indication and that the manufacturing facilities, processes and controls are adequate with respect to such product candidate.
The time required to obtain approval by the FDA and foreign regulatory authorities is unpredictable but typically takes many years following the commencement of preclinical studies and clinical trials and depends upon numerous factors, including the substantial discretion of the regulatory authorities. In addition, approval policies, regulations, or the type and amount of clinical data necessary to gain approval may change during the course of a product candidate’s clinical development and may vary among jurisdictions.
Even if a product candidate were to successfully obtain approval from the FDA and one or more foreign regulatory authorities, any approval might contain significant limitations related to use restrictions for specified age groups, warnings, precautions or contraindications, or may be subject to burdensome post-approval study or risk management requirements. Also, any regulatory approval of our lead product candidate or any future product candidates we may pursue, once obtained, may be withdrawn.
Our lead product candidate and future product candidates could fail to receive regulatory approval from the FDA.
We have not obtained regulatory approval for our lead product candidate, and it is possible that our lead product candidate or any future product candidates will not obtain regulatory approval, for many reasons, including:
| ● | disagreement with the regulatory authorities regarding the scope, design or implementation of our clinical trials; | |
| ● | failure to demonstrate that a product candidate is safe and effective for our proposed indication; | |
| ● | failure of clinical trials to meet the level of statistical significance required for approval; | |
| ● | failure to demonstrate that a product candidate’s clinical and other benefits outweigh its safety risks; | |
| ● | disagreement with our interpretation of data from preclinical studies or clinical trials; | |
| ● | the insufficiency of data collected from clinical trials of our lead product candidate to support the submission and filing of an NDA or other submission or to obtain regulatory approval; | |
| ● | failure to obtain approval of our manufacturing processes or facilities of third party manufacturers with whom we contract for clinical and commercial supplies or our own manufacturing facility; or | |
| ● | changes in the approval policies or regulations that render our preclinical and clinical data insufficient for approval. |
| -25- |
The FDA or a foreign regulatory authority might require more information, including additional preclinical or clinical data, to support approval or additional studies, which might delay or prevent approval or our commercialization plans, or we might decide to abandon the development program. The FDA or a foreign regulatory authority might also require the manufacture of a new lead product candidate in accordance with new or revised standards. If we were to obtain approval, regulatory authorities might approve our lead product candidate and any future product candidates we might pursue for fewer or more limited indications than we request (including failing to approve the most commercially promising indications), might grant approval contingent on the performance of costly post-marketing clinical trials, or might approve a product candidate with a label that does not include the labeling claims necessary or desirable for the successful commercialization of that product candidate.
If we are unable to obtain regulatory approval for our lead product candidate in one or more jurisdictions, or if any approval contains significant limitations, we might not be able to obtain sufficient funding to continue the development of that product or generate revenues attributable to that product candidate.
Failure to obtain regulatory approval in international jurisdictions would prevent our lead product candidate from being marketed abroad.
In addition to regulations in the United States, to market and sell our lead product candidate in the European Union, in the United Kingdom, in many Asian countries and in other jurisdictions, we must obtain separate regulatory approvals and comply with numerous and varying regulatory requirements. Approval by the FDA does not ensure approval by regulatory authorities in other countries or jurisdictions, and approval by one regulatory authority outside the United States does not ensure approval by regulatory authorities in other countries or jurisdictions or by the FDA. The regulatory approval process outside the United States generally includes all of the risks associated with obtaining FDA approval as well as risks attributable to the satisfaction of local regulations in foreign jurisdictions. The approval procedure varies among countries and can require additional data or involve additional testing. The time required to obtain foreign approval may differ substantially from that required to obtain FDA approval. We might not be able to obtain approvals from regulatory authorities outside the United States on a timely basis, if at all. Clinical trials accepted in one country might not be accepted by regulatory authorities in other countries. In addition, many countries outside the United States require that a product be approved for reimbursement before it can be approved for sale in that country. A product candidate that has been approved for sale in a particular country might not receive reimbursement approval in that country.
We might not be able to file for regulatory approvals and might not receive necessary approvals to commercialize our intended product in any market. If we are unable to obtain approval of any of our current product candidate or any future product candidates we might pursue by regulatory authorities in the European Union, United Kingdom, Asia or elsewhere, the commercial prospects of that product candidate might be significantly diminished, our business prospects could decline and this could materially adversely affect our business, results of operations and financial condition.
Even if our current primary product candidate received regulatory approval, it might still face future development and regulatory difficulties.
Even if we obtain regulatory approval for our lead product candidate, LB-100, that approval would be subject to ongoing requirements by the FDA and foreign regulatory authorities governing the manufacture, quality control, further development, labeling, packaging, storage, distribution, adverse event reporting, safety surveillance, import, export, advertising, promotion, recordkeeping and reporting of safety and other post-marketing information. These requirements can include submissions of safety and other post-marketing information and reports, registration, as well as continued compliance by us and/or our CMOs and CROs for any post-approval clinical trials that we or our collaborators might conduct. The safety profile of any product will continue to be closely monitored by the FDA and foreign regulatory authorities after approval. If the FDA or foreign regulatory authorities become aware of new safety information after approval of our lead product candidate, they might require labeling changes or establishment of a risk evaluation and mitigation strategy, impose significant restrictions on such product’s indicated uses or marketing or impose ongoing requirements for potentially costly post-approval studies or post-market surveillance.
| -26- |
In addition, manufacturers of drug products and their facilities are subject to continual review and periodic inspections by the FDA and other regulatory authorities for compliance with CGMP, GCP, and other regulations. If we, a collaborator or a regulatory agency discover previously unknown problems with a product, such as adverse events of unanticipated severity or frequency, or problems with the facility where the product is manufactured, a regulatory agency might impose restrictions on that product, the manufacturing facility or us, including requiring recall or withdrawal of the product from the market or suspension of manufacturing. If we, our lead product candidate or the manufacturing facilities for our lead product candidate fail to comply with applicable regulatory requirements, a regulatory agency might:
| ● | issue warning letters or untitled letters; | |
| ● | mandate modifications to promotional materials or require us to provide corrective information to healthcare practitioners; | |
| ● | require us to enter into a consent decree, which can include imposition of various fines, reimbursements for inspection costs, required due dates for specific actions and penalties for noncompliance; | |
| ● | seek an injunction or impose civil or criminal penalties or monetary fines; | |
| ● | suspend or withdraw regulatory approval; | |
| ● | suspend any ongoing clinical trials; | |
| ● | refuse to approve pending applications or supplements to applications filed by us or a collaborator; | |
| ● | suspend or impose restrictions on operations, including costly new manufacturing requirements; or | |
| ● | seize or detain products, refuse to permit the import or export of products, or require us to initiate a product recall. |
The occurrence of any event or penalty described above might inhibit our ability to successfully commercialize our intended product and generate revenues.
Advertising and promotion of any product candidate that obtains approval in the United States is heavily scrutinized by the FDA, the Department of Justice, the Office of Inspector General of Health and Human Services, state attorneys general, members of Congress and the public. A company can make only those claims relating to safety and efficacy, purity and potency that are approved by the FDA and in accordance with the provisions of the approved label. Additionally, advertising and promotion of any product candidate that obtains approval outside of the United States is heavily scrutinized by foreign regulatory authorities. Violations, including actual or alleged promotion of our intended product for unapproved or off-label uses, are subject to enforcement letters, inquiries and investigations, and civil and criminal sanctions by the FDA, as well as prosecution under the federal False Claims Act. Any actual or alleged failure to comply with labeling and promotion requirements can have a negative impact on our business.
Risks Related to Our Dependence on Third Parties
We depend on certain key scientific personnel for our success who do not work full time for us. The loss of any such personnel could adversely affect our business, financial condition and results of operations.
Effective September 26, 2023, Bas van der Baan, a director of the Company since June 17, 2022, replaced the Company’s founder, Dr. John S. Kovach, as President and Chief Executive Officer. Dr. Kovach passed away on October 5, 2023. Effective October 6, 2023, Mr. van der Baan was appointed as Chairman of the Board of Directors. Dr. Kovach was also the Company’s Chief Scientific Officer.
Although our success depended, in part, on the continued availability and contributions of Dr. Kovach, we were able to replace Dr. Kovach on a timely basis with a qualified replacement in Mr. van der Baan. Furthermore, recruiting and retaining qualified scientific personnel to perform future research and development work is critical to our success. Our inability to attract or retain qualified personnel or advisors in the future could significantly weaken our management, harm our ability to compete effectively, and harm our business. The competition for qualified personnel in the pharmaceutical field is intense and, as a result, we might be unable to attract and retain qualified personnel necessary for the development of our business.
| -27- |
Additionally, we replaced our previous Chief Medical Officer, Dr. James S. Miser, with Dr. Jan Schellens during 2024, and we have reallocated the responsibilities of Eric J. Forman, our Vice President and Chief Operating Officer, who resigned on December 31, 2024. We believe that Mr. Van der Baan and Dr. Schellens are capable of managing the Company’s research and clinical activities.
We expect to rely heavily on third parties for the conduct of clinical trials of our product candidates. If these clinical trials are not successful, or if we or our collaborators are not able to obtain the necessary regulatory approvals, we will not be able to commercialize our product candidates.
In order to obtain regulatory approval for the commercial sale of our product candidates, we or our collaborators will be required to complete extensive preclinical studies as well as clinical trials in humans to demonstrate to the FDA and foreign regulatory authorities that our product candidates are safe and effective.
Dr. Miser is experienced in the design and conduct of early stage clinical trials. However, we expect to rely on collaborative partners and CROs for their performance and management of clinical trials of our product candidates.
Our intended products under development might not be effective in treating any of our targeted disorders or might prove to have undesirable or unintended side effects, toxicities or other characteristics that might prevent or limit their commercial use. Institutional review boards or regulators, including the FDA, might hold, suspend or terminate our clinical research or the clinical trials of our product candidates for various reasons, including non-compliance with regulatory requirements or if, in their opinion, the participating subjects are being exposed to unacceptable health risks. Additionally, failure of third parties conducting or overseeing the operation of the clinical trials to perform their contractual or regulatory obligations in a timely fashion could delay the clinical trials. Failure of clinical trials can occur at any stage. Any of these events would adversely affect our ability to market a product candidate.
The development process necessary to obtain regulatory approval is lengthy, complex and costly. If we or our collaborative partners do not obtain necessary regulatory approvals, then our business would not be successful, and the market price of our common stock could decline substantially.
To the extent that we, or our collaborative partners, are able to successfully advance a product candidate through the clinic, we, or such partner, will be required to obtain regulatory approval prior to marketing and selling such product. The process of obtaining FDA and other required regulatory approvals is costly and lengthy. The time required for FDA and other approvals is uncertain and can typically take several or many years, depending on the complexity and novelty of the product.
Any regulatory approval to market a product might be subject to limitations on the indicated uses for which we, or our collaborative partners, may market the product. These limitations might restrict the size of the market for the product and affect reimbursement by third party payors. In addition, regulatory agencies might not grant approvals on a timely basis or might revoke or significantly modify previously granted approvals.
We, or our collaborative partners, also are subject to numerous foreign regulatory requirements governing the manufacturing and marketing of our potential future products outside of the United States. The approval procedure varies among countries, additional testing might be required in some jurisdictions, and the time required to obtain foreign approvals often differs from that required to obtain FDA approvals. Moreover, approval by the FDA does not ensure approval by regulatory authorities in other countries, and vice versa.
As a result of these factors, we, or our collaborative partners, might not successfully complete clinical trials in the time periods estimated, if at all. Moreover, if we, or our collaborative partners, incur unanticipated costs and/or delays in development programs or if we fail to successfully develop and commercialize products based upon our technologies, we might not be able to generate significant operating revenues or sustainable profitability, as a result of which our stock price could decline substantially.
| -28- |
Business interruptions could adversely affect future operations, revenues, and financial conditions, and might increase our costs and expenses.
Our operations, and those of our directors, advisors, contractors, consultants, CROs, and collaborators, could be adversely affected by earthquakes, floods, hurricanes, typhoons, extreme weather conditions, fires, water shortages, power failures, business systems failures, medical epidemics and other natural and man-made disaster or business interruptions. Our phones, electronic devices and computer systems and those of our directors, advisors, contractors, consultants, CROs, and collaborators are vulnerable to damages, theft and accidental loss, negligence, unauthorized access, terrorism, war, electronic and telecommunications failures, and other natural and man-made disasters. Operating as a virtual company, our employees conduct business outside of our headquarters and leased or owned facilities. These locations might be subject to additional security and other risk factors due to the limited control of our employees. If such an event as described above were to occur in the future, it might cause interruptions in our operations, delay research and development programs, clinical trials, regulatory activities, manufacturing and quality assurance activities, sales and marketing activities, hiring, training of employees and persons within associated third parties, and other business activities. For example, the loss of clinical trial data from completed or future clinical trials could result in delays in our regulatory approval efforts and significantly increase our costs to recover or reproduce the data.
Likewise, we will rely on third parties to manufacture our product candidates and conduct clinical trials, and similar events as those described previously relating to their business systems, equipment and facilities could also have a material adverse effect on our business. To the extent that any disruption or security breach were to result in a loss of, or damage to, our data or applications, or misappropriation or disclosure of confidential or proprietary information, we could incur liability and the further development and commercialization of our lead product candidate could be delayed or altogether terminated.
Our failure to find third party collaborators to assist or share in the costs of product development could materially harm our business, financial condition or results of operations.
Our strategy for the development and commercialization of our proprietary product candidates might include the formation of collaborative arrangements with third parties. We have entered into a number of agreements with third parties as described elsewhere in this document. Existing and future collaborators have significant discretion in determining the efforts and resources they apply and might not perform their obligations as expected. Potential third party collaborators include biopharmaceutical, pharmaceutical and biotechnology companies, academic institutions, government agencies and other entities. Third party collaborators may assist us in:
| ● | funding research, preclinical development, clinical trials and manufacturing; | |
| ● | seeking and obtaining regulatory approvals; and | |
| ● | successfully commercializing any future product candidates. |
If we are not able to establish further collaboration agreements, we might be required to undertake product development and commercialization at our own expense. Such an undertaking might limit the number of product candidates that we will be able to develop, significantly increase our capital requirements and place additional strain on our internal resources. Our failure to enter into additional collaborations could materially harm our business, financial condition and results of operations.
In addition, our dependence on licensing, collaboration and other agreements with third parties might subject us to a number of risks. If we fail to comply with our obligations under these agreements, of if one or more third parties allege that we fail to comply, then one or more third parties might terminate the agreements. In this event, we might not be able to develop, manufacture or market our product candidates. This would materially adversely affect our business prospects.
These agreements might not be on terms that prove favorable to us and might require us to relinquish certain rights in our product candidates. To the extent we agree to work exclusively with one collaborator in a particular territory, research area, or therapeutic field of use, our opportunities to collaborate with other entities could be curtailed. Lengthy negotiations with potential new collaborators might lead to delays in the research, development or commercialization of product candidates. The decision by our collaborators to pursue alternative technologies or the failure of our collaborators to develop or commercialize successfully any product candidate to which they have obtained rights from us could materially harm our business, financial condition and results of operations.
| -29- |
In addition, our agreements might not be assignable by us without the consent of the respective other party or parties, which might limit or delay our ability to consummate transactions, adversely impact the value of those transactions, or limit our ability to pursue research, development or other activities.
We might be subject to claims by third parties asserting that our employees, consultants, collaborators contractors or we have misappropriated their intellectual property, or claiming ownership of what we regard as our own intellectual property.
Our employees, consultants, collaborators or contractors have been previously employed at universities or third party pharmaceutical companies, including our actual or possible competitors, and received confidential and proprietary information from them. Although we try to ensure that our employees, consultants, collaborators or contractors do not use the proprietary information or know-how of others in their work for us, we may be subject to claims that these employees, consultants, collaborators or contractors, or we, have used or disclosed intellectual property, including trade secrets or other proprietary information, of any former employer. We might also be subject to claims that former employers or other third parties have an ownership interest in our patents. Litigation may be necessary to defend against these claims. We might not be successful in defending these claims, and if we fail in defending any such claims, in addition to paying monetary damages, we could lose valuable intellectual property rights, such as exclusive ownership of, or right to use, valuable intellectual property. Even if we are successful, litigation could result in substantial cost and reputational loss and be a distraction to our business.
In addition, while it is our policy to require our employees, consultants, collaborators and contractors who may be involved in the development of intellectual property to execute agreements assigning such intellectual property to us, we might be unsuccessful in executing such an agreement with each party who in fact develops intellectual property that we regard as our own. Such assignment agreements might not be self-executing or may be breached, and we might be forced to bring claims against third parties, or defend claims that third parties might bring against us, to determine the ownership of what we regard as our intellectual property.
Risks Related to Our Intellectual Property
We cannot be certain we will be able to obtain patent protection to protect our product candidates and technology.
Our patents and patent applications are owned solely by our wholly-owned subsidiary, Lixte Biotechnology, Inc., except in several instances where they are jointly owned with one of our collaborators.
The patent prosecution process is expensive and time-consuming, and we might not be able to file or prosecute all necessary or desirable patent applications at a reasonable cost or in a timely manner. It is also possible that we will fail to identify patentable aspects of our research or development before it is too late to obtain patent protection. Therefore, these patents and applications might not be prosecuted and enforced in a manner consistent with the best interests of our business.
The patent position of pharmaceutical companies generally is highly uncertain, involves complex legal and factual questions and has in recent years been the subject of much litigation. In addition, the laws of foreign countries might not protect our rights to the same extent as the laws of the United States. Publications of discoveries in the scientific literature often lag behind the actual discoveries, and patent applications in the United States and other jurisdictions are typically not published until after filing, or in some cases not at all. Therefore, we cannot know with certainty whether we were the first to make the inventions claimed in our solely owned or jointly owned patents or pending patent applications, or that we were the first inventors to file for patent protection of such inventions. As a result, the issuance, scope, validity, enforceability and commercial value of our patent rights are highly uncertain. Our pending and future patent applications might not result in patents being issued that protect our technology or products, in whole or in part, or that effectively prevent others from commercializing competitive technologies and products. Changes in the patent laws or their interpretation by courts or patent offices might diminish the value of our patents or patent applications, or narrow their scope.
The issuance of a patent is not conclusive as to its inventorship, scope, term, validity or enforceability, and our solely or jointly owned patents might be challenged in a U.S. or non-U.S. court or patent office. Such challenges might result in loss of exclusivity or in patent claims being narrowed, invalidated or held unenforceable, in whole or in part, which could limit our ability to stop others from using or commercializing similar or identical technology and products, or limit the duration of the patent protection of our technology and products. Given the amount of time required for research, development, testing or regulatory review of product candidates, patents protecting such candidates might expire before or shortly after such candidates are approved or commercialized. As a result, our solely or jointly owned patents might not provide us with sufficient rights to exclude others from commercializing intended products similar or identical to ours.
| -30- |
We cannot be certain that all patents applied for will be issued. If a third party has also filed a patent application relating to an invention claimed by us, solely or jointly with one of our collaborators, we might be required to participate in an interference or derivation proceeding declared or instituted by the United States Patent and Trademark Office, which could result in substantial uncertainties and cost for us, even if the eventual outcome is favorable to us. The degree of future protection for our proprietary rights is uncertain. For example:
| ● | we, solely or jointly with our collaborators, might not have been the first to make the inventions covered by our pending or future patent applications; | |
| ● | we, solely or jointly with our collaborators, might not have been the first to file patent applications for these inventions; | |
| ● | others might independently develop identical, similar or alternative technologies; | |
| ● | it is possible that our patent applications will not result in an issued patent or patents, or that the scope of protection granted by any patents arising from our patent applications will be significantly narrower than expected; |
|
| ● | we might be unaware of prior art that renders one or more of our patent applications unpatentable or one or more of our patents invalid; | |
| ● | a court might determine that we failed to disclose to a patent office prior art that we were aware of and that is material to patentability and, therefore, conclude that one or more of our patents are unenforceable; | |
| ● | any patents under which we hold rights might not cover commercially viable products, might not provide us with any competitive advantages or might be challenged by one or more third parties as being not infringed, being invalid, or being unenforceable under United States or foreign laws; | |
| ● | a court or patent office might determine that two or more of our patents claim patentably indistinct subject matter, which could adversely affect one or more of the patents’ term, validity or enforceability; | |
| ● | a court or patent office might determine that one or more patents issued to us in the future or under which we hold rights are invalid or unenforceable; or | |
| ● | we might develop additional proprietary technologies that are not patentable and which might not be adequately protected through trade secrets or know-how. |
In addition, we solely or jointly own patents or patent applications in jurisdictions having, or that might in the future have, geopolitical disputes, including over sovereignty. We cannot guarantee that patents granted in these jurisdictions will be enforceable. An inability to enforce patents in these jurisdictions could have a material adverse effect on our business.
If we do not obtain patent term extension in the United States under the Hatch-Waxman Act or in foreign countries under similar legislation, our business might be materially harmed.
In the United States, the term of a patent that covers an FDA-approved drug, its method for use or method for manufacture, can be eligible for patent term extension. U.S. law provides a patent term extension of up to five years beyond the expiration of the patent for time during which the drug is under regulatory review. Patent term extension cannot extend the term of a patent beyond a total of 14 years from the date of regulatory approval; only one patent can be extended for the same regulatory review period; and the scope of a patent’s enforceability during a patent term extension is limited to the scope of FDA approval. There is no guarantee that the relevant agencies, including the United States Patent and Trademark Office (“USPTO”), will agree with our assessment of whether such extensions should be granted, and even if granted, the term of these extensions. We might not be granted patent term extension in the United States or in any foreign country because of, for example, expiration of our patents before obtaining regulatory approval, failing to exercise due diligence during the testing phase or regulatory review process, failing to apply within applicable deadlines, failing to apply prior to expiration of relevant patents or otherwise failing to satisfy applicable requirements. Moreover, the length of a patent term extension, as well as the scope of patent protection during any such extension, afforded by the governmental authority could be less than we request. If we are unable to obtain any patent term extension or if the term of any such extension is less than we request, our competitors might obtain approval of competing products following the expiration of our patent rights, and our business, financial condition, results of operations and prospects could be materially harmed.
| -31- |
It is possible that we will not obtain patent term extension under the Hatch-Waxman Act for a U.S. patent covering any of our product candidates that we may identify even where that patent is eligible for patent term extension, or if we obtain such an extension, it may be for a shorter period than we had sought.
If we fail to comply with our obligations in agreements under which we have licensed or, might license, intellectual property rights from third parties, or if we otherwise experience disruptions to our business relationships with our licensors, we could lose rights that are important to our business.
We have entered into, and might in the future enter into, one or more intellectual property license agreements that are important to our business. These license agreements might impose various diligence, milestone payment, royalty and other obligations on us. For example, we might be required to use commercially reasonable efforts to engage in various development and commercialization activities with respect to licensed products, and might need to satisfy specified milestone and royalty payment obligations. If we fail to comply with any obligations under our agreements with any of these licensors, we might be subject to termination of the license agreement in whole or in part, increased financial obligations to our licensors or loss of exclusivity in a particular field or territory, in which case our ability to develop or commercialize products covered by the license agreement will be impaired.
In addition, disputes might arise regarding intellectual property subject to a license agreement, including:
| ● | the scope of rights granted under the license agreement and other interpretation-related issues; | |
| ● | whether our technology, product candidates or processes infringe intellectual property rights that are owned by the licensor, but that are not subject to the licensing agreement; | |
| ● | our diligence obligations under the license agreement and the activities that satisfy those obligations; | |
| ● | whether we are required to sublicense to a third party rights that the license grants to us, but that we do not commercially pursue; and | |
| ● | the ownership of inventions, data and know-how resulting from joint creation or use of intellectual property by our licensors and us. |
If disputes over intellectual property that we have licensed, or might in the future license, prevent or impair our ability to maintain our licensing arrangements on acceptable terms, we may be unable to successfully develop or commercialize the affected product candidates.
We might need to obtain licenses from third parties to advance our research or allow commercialization of our product candidates. We may fail to obtain any of these licenses at a commercially reasonable cost or on commercially reasonable terms, if at all. Other companies might have a competitive advantage over us due to their larger size or cash resources or greater clinical development and commercialization capabilities. We might be unable to further develop or commercialize one or more of our product candidates, which could harm our business significantly.
| -32- |
We might infringe the intellectual property rights of others, which may prevent or delay our product development efforts and stop us from commercializing or increase the costs of commercializing our product candidates.
Our success will depend in part on our ability to operate without infringing the proprietary rights of third parties. We cannot guarantee that our intended products or our product candidates, or manufacture or use of our intended products or our product candidates, will not infringe third party patents. Furthermore, a third party might claim that we are using without permission one or more inventions covered by the third party’s patent rights and might go to court to stop us from engaging in our normal operations and activities, including making, offering to sell or selling our product candidates. Still further a third party might go to court seeking judgment that our patents are invalid or unenforceable. These lawsuits are costly and could affect our results of operations and divert the attention of managerial and scientific personnel. Some of these third parties might be better capitalized and have more resources than us. There is a risk that a court would decide that we are infringing the third party’s patents and would order us to stop the activities covered by the patents. In that event, we might not have a viable way around the patent and might need to halt commercialization of the relevant product candidate. In addition, there is a risk that a court will order us to pay the other party damages for having violated the other party’s patents. There is also a risk that a court would decide that one or more of our patents are invalid or unenforceable. In addition, we might be obligated to indemnify our licensors and collaborators against intellectual property infringement claims brought by third parties, which could require us to expend additional resources. The pharmaceutical and biotechnology industries have produced a proliferation of patents, and it is not always clear to industry participants, including us, which patents cover various types of products or methods of use. The coverage of patents is subject to interpretation by the courts, and the interpretation is not always uniform.
We cannot guarantee that we have identified all third party patents or pending patent applications that are or might be necessary for the commercialization of our intended products and technologies in any jurisdiction. Patent applications in the United States and elsewhere are not published until approximately 18 months after the earliest filing for which priority is claimed, with such earliest filing date being commonly referred to as the priority date. Therefore, patent applications covering our technologies and intended products could have been filed by others without our knowledge.
Additionally, pending patent applications that have been published can, subject to certain limitations, be later amended in a manner that could cover our technologies or intended products. The scope of a patent claim is determined by the interpretation of the law, the words of a patent claim, the written disclosure in a patent and the patent’s prosecution history. Our interpretation of the relevance or the scope of a patent or a pending patent application may be incorrect, which may negatively impact our ability to market our intended products. We might incorrectly determine that our technologies or intended products are not covered by a third party patent or might incorrectly predict whether a third party’s pending patent application will issue with claims of relevant scope. Our determination of the expiration date of any patent in the United States or abroad that we consider relevant might be incorrect, and we might incorrectly conclude that a third party patent does not cover our technology or intended products, is invalid or is unenforceable. Our inability to identify or correctly interpret relevant patents might negatively impact our ability to develop or market our technologies or intended products. If we fail to identify or correctly interpret relevant patents, we might be subject to infringement claims. We cannot guarantee that we will be able to successfully settle or otherwise resolve such infringement claims. If we fail in any such dispute, in addition to being liable for damages, we might be temporarily or permanently enjoined or otherwise prohibited from commercializing any of technologies or intended products that are held to be infringing. We might, if possible, also be forced to redesign intended products or product formulations so that we no longer infringe the third party intellectual property rights. Any of these events, even if we were ultimately to prevail, could require us to divert substantial financial and management resources that we would otherwise be able to devote to our business.
As the pharmaceutical or biotechnology industry expands and more patents are issued, the risk increases that our product candidates or intended products give rise to claims of infringement of the patent rights of others. There may be third party patents of which we are currently unaware with claims to materials, formulations, methods of manufacture or methods for treatment related to the use or manufacture of our product candidates. If we are sued for patent infringement, we would need to demonstrate that our products or methods either do not infringe the patent claims of the relevant patent or that the patent claims are invalid or unenforceable, negotiate and obtain a license under reasonable terms to us or discontinue performing the allegedly infringing activities. We might not be able to do any of these. Proving invalidity is difficult. For example, in the United States, proving invalidity requires a showing of clear and convincing evidence to overcome the presumption of validity enjoyed by issued patents. Even if we are successful in these proceedings, we might incur substantial costs and divert management’s time and attention in pursuing these proceedings, which could have a material adverse effect on us. If we are unable to avoid infringing the patent rights of others, we might be required to seek a license, which might not be available, and then we will have to defend an infringement action, challenge the validity of the patents in the USPTO or in court, or discontinue performing the allegedly infringing activities. Patent litigation is costly and time consuming. We might not have sufficient resources to bring these actions to a successful conclusion. In addition, if we do not obtain a license, develop or obtain non-infringing technology, fail to defend an infringement action successfully or fail to have infringed patents declared invalid or unenforceable, we might incur substantial monetary damages, encounter significant delays in bringing our product candidates to market and be precluded from manufacturing or selling our product candidates.
| -33- |
We cannot be certain that others have not filed patent applications for technology covered by our pending applications, that we were the first to invent the technology or that we were the first to file patent applications covering our technology, because:
| ● | some patent applications in the United States are maintained in secrecy until the patents are issued; | |
| ● | patent applications in the United States are typically not published until 18 months after their earliest claimed priority date; and | |
| ● | publications in the scientific literature often lag behind actual discoveries. |
Our competitors might have filed, and might in the future file, patent applications covering technology similar or identical to ours. Any such patent applications might dominate our patent applications, which could further require us to obtain rights to issued patents covering such technologies. If another party has filed US patent applications that cover inventions similar or identical to ours and claim priority to any applications filed prior to the priority dates of our applications, we might have to participate in an interference proceeding declared or a derivation proceed instituted by the USPTO to determine priority of invention in the United States. The costs of these proceedings could be substantial, and it is possible that such efforts would be unsuccessful if the other party had independently arrived at the same or similar inventions before us, possibly resulting in a loss of our U.S. patent position with respect to such inventions. Other countries might have similar laws that permit secrecy of patent applications. Either way, the third party’s patents or patent applications might be entitled to priority over our applications in such jurisdictions.
Some of our competitors might be able to sustain the costs of a patent challenge more effectively than we can because they have substantially greater resources. In addition, uncertainties regarding the outcome of the challenge could have a material adverse effect on our ability to raise the funds necessary to continue our operations.
We might be subject to claims that our employees, consultants or independent contractors have wrongfully used or disclosed alleged trade secrets of one or more third parties.
As is common in the biotechnology and pharmaceutical industries, we employ, and might employ in the future, individuals who were previously employed at other biotechnology or pharmaceutical companies, including our competitors or potential competitors. Although we try to ensure that our employees, consultants and independent contractors do not use the proprietary information or know-how of others in their work for us, we might be subject to claims that we or our employees, consultants or independent contractors have inadvertently or otherwise used or disclosed trade secrets or other proprietary information of their former employers. Litigation might be necessary to defend against these claims. If we fail in defending any such claims, in addition to paying monetary damages, we could lose valuable intellectual property rights or personnel, which could adversely impact our business. Even if we are successful in defending against these claims, litigation could result in substantial costs and be a distraction to management.
Our intellectual property might not be sufficient to protect our intended products from competition, which might negatively affect our business as well as limit our partnership or acquisition appeal.
We might be subject to competition despite the existence of intellectual property we license or own. We can give no assurance that our intellectual property claims will be sufficient to prevent third parties from designing around patents we own or license and developing and commercializing competitive products. The existence of competitive products that avoid our intellectual property could materially adversely affect our operating results and financial condition. Furthermore, any actual or perceived limitations, in our intellectual property might lessen the interest of third parties to partner, collaborate or otherwise transact with us, if third parties perceive a higher than acceptable risk to commercialization of our intended products or future products.
| -34- |
Our approach includes filing patent applications covering combination therapy with known, studied and/or marketed drugs. Although the protection afforded by our patent applications might be significant, when looking at our patents’ ability to block competition, the protection offered by our patents might be, to some extent, more limited than protection provided by patents claiming a composition of matter that is entirely new and previously unknown. If a competitor were able to successfully design around any combination therapy patents we have or might have in the future, our business and competitive advantage could be significantly affected.
We might elect to sue a third party, or otherwise make a claim, alleging infringement or other violation of patents, trademarks, trade dress, copyrights, trade secrets, domain names or other intellectual property rights that we either own or license. We might alternatively elect to sue a third party, or otherwise make a claim, alleging that we don’t infringe a third party’s patents or that the third party’s patents are invalid or unenforceable. Any claims that we assert against a third party could provoke the third party to assert one or more counterclaims against us, for example, alleging that we infringe their patents. In addition, in a patent infringement proceeding, a court might decide that a patent of ours is invalid or unenforceable, in whole or in part; construe the patent’s claims narrowly; or refuse to stop the other party from using the technology at issue. Any litigation proceeding could put one or more of our patents at risk of being invalidated, held unenforceable or interpreted narrowly. Even if we prevail in a lawsuit, a court might not award remedies that sufficiently compensate us for our losses.
If we do not prevail in either type of litigation, we might be subject to:
| ● | paying monetary damages related to the legal expenses of the third party; | |
| ● | facing additional competition that might have a significant adverse effect on our intended-product pricing, market share, business operations, financial condition, and the commercial viability of our intended products; and | |
| ● | restructuring our company or delaying or terminating select business opportunities, including, but not limited to, research and development, clinical trials, and commercialization activities, due to a potential deterioration of our financial condition or market competitiveness. |
A third party might also challenge the validity, enforceability or scope of the intellectual property rights that we license or own, and the result of these challenges might narrow the scope or claims of or invalidate patents that are integral to our product candidates in the future. There can be no assurance that we will be able to successfully defend patents we own in an action against third parties due to the unpredictability of litigation and the high costs associated with intellectual property litigation, among other factors.
The laws of some jurisdictions do not protect intellectual property rights to the same extent as the laws or rules and regulations of the United States, and many companies have encountered significant difficulties in protecting and defending such rights in non-U.S. jurisdictions. The legal systems of some countries are less supportive of enforcement of patents, trade secrets and other intellectual property protection, than the United States. This could make it difficult for us to enforce our patents or market competing products outside the United States, in violation of our proprietary rights generally. Proceedings to enforce our patent rights in non-U.S. jurisdictions, whether or not successful, could result in substantial costs and divert our efforts and attention from other aspects of our business, could put our patents at risk of being invalidated or interpreted narrowly and our patent applications at risk of not issuing, and could provoke third parties to assert claims against us. We might not prevail in any lawsuits that we initiate, and the damages or other remedies awarded, if any, might not be commercially meaningful. Accordingly, our efforts to enforce our intellectual property rights in all jurisdictions where we have the rights might be inadequate to obtain a significant commercial advantage from the intellectual property that we develop or license. Furthermore, while we seek to protect our intellectual property rights in significant markets, we cannot ensure that we will be able to initiate or maintain similar efforts in all jurisdictions in which we might wish to market our intended products or our product candidates. Accordingly, our efforts to protect our intellectual property rights in such countries might be inadequate, which might have an adverse effect on our ability to successfully commercialize our product candidates in all of our expected significant foreign markets. If we or our licensors encounter difficulties in protecting, or are otherwise precluded from effectively protecting, the intellectual property rights important for our business in such jurisdictions, the value of these rights might be diminished, and we might face additional competition from others in those jurisdictions.
| -35- |
Changes to patent law, for example the Leahy-Smith America Invests Act, AIA or Leahy-Smith Act, of 2011 and the Patent Reform Act of 2009 and other future article of legislation in the U.S., might substantially change the regulations and procedures surrounding patent applications, issuance of patents, prosecution of patents, challenges to patent validity, and patent enforcement. We can give no assurance that our patents or those of our licensor(s) can be defended or will protect us against future intellectual property challenges, particularly as they pertain to changes in patent law and future patent law interpretations.
In addition, enforcing and maintaining our intellectual property protection depends on compliance with various procedural, document-submission, fee-payment and other requirements imposed by the U.S. Patent and Trademark Office and courts, and foreign government patent agencies and courts, and our patent protection could be reduced or eliminated for non-compliance with these requirements.
Filing, prosecuting and defending patents covering our product candidates in all countries throughout the world would be prohibitively expensive, and our intellectual property rights in some jurisdictions outside the United States can be less extensive than those in the United States. And filing, prosecuting and defending patents even in only those jurisdictions in which we develop or commercialize our product candidates might be prohibitively expensive or impractical. Competitors might use our technologies in jurisdictions where we have not obtained patent protection to develop their own products or technologies and, further, may export otherwise infringing products or technologies to territories where we and have patent protection, but where enforcement is not as strong as that in the United States. These third party products or technologies might compete with our product candidates, and our intellectual property rights may not be effective or sufficient to prevent third parties from competing.
In addition, we might decide to abandon national or regional patent applications while they are still pending or to abandon granted patents. This might invite or encourage third parties to develop their products or technologies in jurisdictions where we abandon patent applications or patents.
If we are not able to protect and control our unpatented trade secrets, know-how and other technological innovation, we might suffer competitive harm.
We also rely on proprietary trade secrets and unpatented know-how to protect our research and development activities, particularly when we do not believe that patent protection is appropriate or available. However, trade secrets are difficult to protect. We will attempt to protect our trade secrets and unpatented know-how by requiring our employees, consultants, collaborators, and advisors to execute a confidentiality and non-use agreement. We cannot guarantee that these agreements will provide meaningful protection; that these agreements will not be breached, by, e.g., a misappropriating or disclosing our confidential information; that we will have an adequate remedy for any such breach; or that our trade secrets will not otherwise become known or independently developed by a third party. Our trade secrets, and those of our present or future collaborators that we utilize by agreement, might become known or might be independently discovered by others, which could adversely affect the competitive position of our product candidates.
We might incur substantial costs prosecuting our patent applications, maintaining our patents and patent applications, enforcing our patents, defending against third party patent infringement suits, seeking invalidation of third party patents or in-licensing third party intellectual property, as a result of litigation or other proceedings relating to patent and other intellectual property rights.
We might be unaware of or unfamiliar with prior art and/or interpretations of prior art that could potentially impact the validity or scope of our patents or pending patent applications, or patent applications that we will file. We might have elected, or elect now or in the future, not to maintain or pursue intellectual property rights that, at some point in time, might be considered relevant to or enforceable against a competitor.
We take efforts and enter into agreements with employees, consultants, collaborators, and advisors to confirm ownership of and chain of title in intellectual property rights. However, an inventorship or ownership dispute could arise that might permit one or more third parties to practice our intellectual property rights, including possible efforts to enforce rights against us.
| -36- |
We might not have rights under some patents or patent applications that cover technologies that we use in our research, drug targets that we select, product candidates and particular uses thereof that we seek to develop and commercialize, as well as synthesis of our product candidates. Third parties might own or control these patents and patent applications in the United States and elsewhere. These third parties could bring claims against us or our collaborators that would cause us to incur substantial expenses and, if successful against us, could cause us to pay substantial damages. Further, if a patent infringement suit were brought against us or our collaborators, we or they could be forced to stop or delay research, development, manufacturing or sales of the product or product candidate that is the subject of the suit. We or our collaborators therefore might choose to seek, or be required to seek, a license from the third party and would most likely be required to pay license fees or royalties or both. These licenses might not be available on acceptable terms, or at all. Even if we or our collaborators were able to obtain a license, the rights might be nonexclusive, which would give our competitors access to the same intellectual property. Ultimately, we could be prevented from commercializing a product or product candidate, or forced to cease some aspect of our business operations, as a result of patent infringement claims, which could harm our business.
Periodic maintenance fees on issued U.S. patents are due to be paid to the USPTO, and periodic maintenance fees on issued non-U.S. patents and pending non-U.S. patent applications are due to be paid to non-U.S. patent offices. Patent offices require compliance with many procedural, documentary, fee payment and other requirements during the patent application process and after a patent issues or grants. While an inadvertent lapse can in some cases be cured by payment of a late fee or by other means in accordance with the applicable rules, there are situations in which non-compliance, for example, caused by geopolitical events such as civil or political unrest (including the ongoing conflict between Ukraine and Russia), can result in abandonment or lapse of the patent or patent application, resulting in partial or complete loss of a patent or patent rights in the relevant jurisdiction. Non-compliance events that could result in abandonment or lapse of a patent or patent application include, but are not limited to, failure to respond to patent office actions within prescribed time limits, non-payment of fees and failure to properly legalize and submit formal documents. In such an event, our competitors might be able to enter the market, which would have a material adverse effect on our business.
The USPTO and various non-U.S. government agencies require compliance with certain foreign filing requirements during the patent application process. For example, in some countries, including the United States, a foreign filing license is required before certain patent applications are filed outside that country. The foreign filing license requirements can vary by country. In some cases, a foreign filing license may be obtained retroactively in accordance with the applicable rules. There are situations, however, in which non-compliance can result in abandonment of a pending patent application or can be grounds for revoking or invalidating an issued patent, resulting in the loss of patent rights in the relevant jurisdiction. In such an event, potential competitors might be able to enter the relevant markets with similar or identical products or technology, which could have a material adverse effect on our business, financial condition, results of operations, and prospects.
There has been substantial litigation and other legal proceedings regarding patent and other intellectual property rights in the pharmaceutical and biotechnology industries. Although we are not currently a party to any patent litigation or any other adversarial proceeding, including any interference or derivation proceeding declared or instituted before the United States Patent and Trademark Office, regarding intellectual property rights with respect to our intended products, our product candidates and our technology, it is possible that we might become one in the future. We are not currently aware of any actual or reasonably foreseeable third party infringement claim involving our product candidates. The cost to us of any patent litigation or other proceeding, even if resolved in our favor, could be substantial. The outcome of patent litigation is subject to uncertainties that cannot be adequately quantified in advance, including the dispute forum, demeanor and credibility of witnesses and the identity of the adverse party, especially in pharmaceutical and biotechnology related patent cases that might turn on the testimony of experts as to technical facts upon which experts might reasonably disagree. Some of our competitors might be able to sustain the costs of such litigation or proceedings more effectively than we can because of their substantially greater financial resources. If a patent or other proceeding is resolved against us, we might be enjoined from researching, developing, manufacturing or commercializing our intended products or our product candidates without a license from the other party and we might be held liable for significant damages. We might not be able to obtain any required license on commercially acceptable terms or at all.
Uncertainties resulting from the initiation and continuation of patent litigation or other proceedings could harm our ability to compete in the marketplace. Patent litigation or other proceedings might also absorb significant management time.
| -37- |
If we are unable to protect our intellectual property rights, our competitors might develop and market products with similar or identical features that might reduce demand for our potential products.
The following factors are important to our success:
| ● | receiving patent protection for our product candidates; | |
| ● | preventing others from infringing our intellectual property rights; and | |
| ● | maintaining our patent rights and trade secrets. |
We will be able to protect our intellectual property rights in patents and trade secrets from unauthorized use by third parties only to the extent that such intellectual property rights are covered by valid and enforceable patents or are effectively maintained as trade secrets and we enforce these rights.
Because issues of patentability involve complex legal and factual questions, the issuance, scope or enforceability of patents cannot be predicted with certainty. Patents can be challenged, invalidated, found unenforceable, or circumvented. United States patents and patent applications can be subject to interference or derivation proceedings. United States patents can also be subject to post grant proceedings, including re-examination, derivation, Inter Partes Review and Post Grant Review, in the United States Patent and Trademark Office. Foreign patents can be subject to opposition or comparable proceedings in corresponding foreign patent offices. Any of these challenges might result in loss of the patent, rejection of the patent application or loss or reduction in the scope of one or more of the claims of the patent or patent application. In addition, these proceedings can be costly. Thus, any patents that we own or license from others might not provide any protection against competitors. Furthermore, an adverse decision in an interference or derivation proceeding can result in a third party receiving the patent rights sought by us, which in turn could affect our ability to market a potential product to which that patent filing was directed. Our pending patent applications, those that we might file in the future, or those that we might license from third parties might not result in patents being issued. If issued, they might not provide us with proprietary protection or competitive advantages against competitors with similar or identical technology. Furthermore, others might independently develop similar technologies or duplicate any technology that we have developed. Some countries have compulsory licensing laws under which a patent owner might be compelled to grant licenses to third parties. For example, compulsory licenses might be required in cases such as where the patent owner has failed to “work” the invention in that country or where a third party has patented improvements. In addition, some countries might limit the enforceability of patents against government agencies or government contractors. In these countries, we might have limited infringement remedies, which could materially diminish the value of our patents. Moreover, the legal systems of some countries are less supportive of enforcement of patents, trade secrets and other intellectual property protection, than the United States, which might make it difficult to stop infringement in these countries.
In addition, our ability to enforce our patent rights depends on our ability to detect infringement. It is difficult to detect infringers who do not advertise or otherwise promote the compounds that are used in their products. Any litigation to enforce or defend our patent rights, even if we prevail, could be costly and time-consuming and would divert the attention of management and key personnel from business operations.
We will also rely on trade secrets, know-how and technology, which are not protected by patents, to maintain our competitive position. We will seek to protect this information by entering into confidentiality agreements with parties that have access to it, such as strategic partners, collaborators, employees, contractors and consultants. Any of these parties might breach these agreements and misappropriate or disclose our confidential information or our competitors might learn of the information in some other way. If any trade secret, know-how or other technology not protected by a patent were disclosed to, or independently developed by, a competitor, our business, financial condition and results of operations could be materially adversely affected.
Risks Related to Commercialization of Our Current Product Candidate and Future Product Candidates
Our commercial success depends upon attaining significant market acceptance of our current product candidate and future product candidates, if approved, among physicians, patients, healthcare payors and cancer treatment centers.
Even if we obtain regulatory approval for our lead product candidate or any future product candidates, the products might not gain market acceptance among physicians, healthcare payors, patients or the medical community, including cancer treatment centers. Market acceptance of any product candidates for which we receive approval depends on a number of factors, including:
| ● | the efficacy and safety of such product candidates as demonstrated in clinical trials; |
| -38- |
| ● | the clinical indications and patient populations for which the product candidate is approved; | |
| ● | acceptance by physicians, major cancer treatment centers and patients of the drug as a safe and effective treatment; | |
| ● | the adoption of novel immunotherapies by physicians, hospitals and third party payors; | |
| ● | the potential and perceived advantages of product candidates over alternative treatments; | |
| ● | the safety of product candidates seen in a broader patient group, including our use outside the approved indications; | |
| ● | any restrictions on use together with other medications; | |
| ● | the prevalence and severity of any side effects; | |
| ● | product labeling or product insert requirements of the FDA or other regulatory authorities; | |
| ● | the timing of market introduction of our intended product as well as competitive products; | |
| ● | the development of manufacturing and distribution processes for commercial scale manufacturing for our lead product candidate and any future product candidates; | |
| ● | the cost of treatment in relation to alternative treatments; | |
| ● | the availability of coverage and adequate reimbursement from third party payors and government authorities; | |
| ● | relative convenience and ease of administration; and | |
| ● | the effectiveness of our sales and marketing efforts and those of our collaborators. |
If our lead product candidate and any future product candidates are approved but fail to achieve market acceptance among physicians, patients, healthcare payors or cancer treatment centers, we will not be able to generate significant revenues, which would compromise our ability to become profitable.
Even if we are able to commercialize our lead product candidate or any future product candidates, the products might not receive coverage or adequate reimbursement from third party payors in the United States and in other countries in which we seek to commercialize our intended products, which could harm our business.
Our ability to commercialize any product successfully will depend, in part, on the extent to which coverage and adequate reimbursement for such product and related treatments will be available from third party payors, including government health administration authorities, private health insurers and other organizations.
Third party payors determine which medications they will cover and establish reimbursement levels. A primary trend in the healthcare industry is cost containment. Third party payors have attempted to control costs by limiting coverage and the amount of reimbursement for particular medications. Increasingly, third party payors are requiring that drug companies provide them with predetermined discounts from list prices and are challenging the prices charged for medical products. Third party payors might also seek additional clinical evidence, beyond the data required to obtain regulatory approval, demonstrating clinical benefit and value in specific patient populations before covering our intended product for those patients. We cannot be sure that coverage and adequate reimbursement will be available for any product that we commercialize and, if coverage is available, what the level of reimbursement will be. Coverage and reimbursement might impact the demand for, or the price of, any product candidate for which we obtain regulatory approval. If reimbursement is not available or is available only at limited levels, we might not be able to successfully commercialize any product candidate for which we obtain regulatory approval.
| -39- |
There might be significant delays in obtaining coverage and reimbursement for newly approved drugs, and coverage might be more limited than the purposes for which the drug is approved by the FDA or comparable foreign regulatory authorities. Moreover, eligibility for coverage and reimbursement does not imply that any drug will be paid for in all cases or at a rate that covers our costs, including research, development, manufacture, sale and distribution. Interim reimbursement levels for new drugs, if applicable, might also not be sufficient to cover our costs and might only be temporary. Reimbursement rates might vary according to the use of the drug and the clinical setting in which it is used, might be based on reimbursement levels already set for lower cost drugs and might be incorporated into existing payments for other services. Net prices for drugs might be reduced by mandatory discounts or rebates required by third party payors and by any future relaxation of laws that presently restrict imports of drugs from countries where they might be sold at lower prices than in the United States. No uniform policy for coverage and reimbursement exists in the United States, and coverage and reimbursement can differ significantly from payor to payor. Third party payors can rely upon Medicare coverage policy and payment limitations in setting their own reimbursement policies, but also have their own methods and approval process apart from Medicare determinations. Our inability to promptly obtain coverage and profitable reimbursement rates from both government-funded and private payors for any approved product that we develop could have a material adverse effect on our operating results, ability to raise capital needed to commercialize our intended product and overall financial condition.
Healthcare legislative measures aimed at reducing healthcare costs might have a material adverse effect on our business and results of operations.
Third party payors, whether domestic or foreign, or governmental or commercial, are developing increasingly sophisticated methods of controlling healthcare costs. In both the United States and certain international jurisdictions, there have been a number of legislative and regulatory changes to the health care system that could impact our ability to sell our intended product profitably. In particular, in 2010, the Affordable Care Act (“ACA”) was enacted, which, among other things, subjected biologic products to potential competition by lower-cost biosimilars, addressed a new methodology by which rebates owed by manufacturers under the Medicaid Drug Rebate Program are calculated for drugs that are inhaled, infused, instilled, implanted or injected, increased the minimum Medicaid rebates owed by most manufacturers under the Medicaid Drug Rebate Program, extended the Medicaid Drug Rebate Program to utilization of prescriptions of individuals enrolled in Medicaid managed care organizations, subjected manufacturers to new annual fees and taxes for certain branded prescription drugs, and provided incentives to programs that increase the federal government’s comparative effectiveness research.
There have been, and likely will continue to be, legislative and regulatory proposals at the foreign, federal and state levels directed at containing or lowering the cost of healthcare. We cannot predict the initiatives that might be adopted in the future. The continuing efforts of the government, insurance companies, managed care organizations and other payors of healthcare services to contain or reduce costs of healthcare and/or impose price controls might adversely affect:
| ● | the demand for our lead product candidate, if we obtain regulatory approval; | |
| ● | our ability to receive or set a price that we believe is fair for our intended product; | |
| ● | our ability to generate revenue and achieve or maintain profitability; | |
| ● | the level of taxes that we are required to pay; and | |
| ● | the availability of capital. |
We expect that the ACA, as well as other healthcare reform measures that might be adopted in the future, might result in additional reductions in Medicare and other healthcare funding, more rigorous coverage criteria, lower reimbursement and new payment methodologies. This could lower the price that we receive for any approved product. Any denial in coverage or reduction in reimbursement from Medicare or other government-funded programs might result in a similar denial or reduction in payments from private payors, which might prevent us from being able to generate sufficient revenue, attain profitability or commercialize our product candidate, if approved.
| -40- |
Price controls might be imposed in foreign markets, which might adversely affect our future profitability.
In some countries, the pricing of prescription drugs is subject to governmental control. In these countries, pricing negotiations with governmental authorities can take considerable time after receipt of regulatory approval for a product. In addition, there can be considerable pressure by governments and other stakeholders on prices and reimbursement levels, including as part of cost containment measures. Political, economic and regulatory developments might further complicate pricing negotiations, and pricing negotiations might continue after reimbursement has been obtained. Reference pricing used by various European Union member states and parallel distribution, or arbitrage between low-priced and high-priced member states, can further reduce prices.
In some countries, we or our collaborators might be required to conduct a clinical trial or other studies that compare the cost-effectiveness of our product candidate to other available therapies in order to obtain or maintain reimbursement or pricing approval. Publication of discounts by third party payors or authorities might lead to further pressure on the prices or reimbursement levels within the country of publication and other countries. If reimbursement of our intended product is unavailable or limited in scope or amount, or if pricing is set at unsatisfactory levels, our business could be adversely affected.
Risks Related to Healthcare Compliance Regulations
Our relationships with customers and third party payors will be subject to applicable anti-kickback, fraud and abuse and other healthcare laws and regulations, which could expose us to criminal sanctions, civil penalties, contractual damages, reputational harm and diminished profits and future earnings. If we or they are unable to comply with these provisions, we might become subject to civil and criminal investigations and proceedings that could have a material adverse effect on our business, financial condition and prospects.
Healthcare providers, physicians and third party payors will play a primary role in the recommendation and prescription of any product candidates for which we obtain regulatory approval. Our current and future arrangements with healthcare providers, healthcare entities, third party payors and customers might expose us to broadly applicable fraud and abuse and other healthcare laws and regulations that might constrain the business or financial arrangements and relationships through which we research, develop and will market, sell and distribute our intended product. As a pharmaceutical company, even though we do not and will not control referrals of healthcare services or bill directly to Medicare, Medicaid or other third party payors, federal and state healthcare laws and regulations pertaining to fraud and abuse and patients’ rights are applicable to our business. Restrictions under applicable federal and state healthcare laws and regulations that might affect our ability to operate include the following:
| ● | the federal healthcare Anti-Kickback Statute which prohibits, among other things, individuals and entities from knowingly and willfully soliciting, offering, receiving or providing remuneration, directly or indirectly, overtly or covertly, in cash or in kind, to induce or reward, or in return for, either the referral of an individual for, or the purchase, order or recommendation of, any good or service, for which payment might be made under a federal healthcare program such as Medicare and Medicaid; | |
| ● | federal civil and criminal false claims laws, including the federal False Claims Act that can be enforced through civil whistleblower or qui tam actions, and civil monetary penalty laws, prohibit individuals or entities from knowingly presenting, or causing to be presented, to the federal government, including the Medicare and Medicaid programs, claims for payment or approval that are false or fraudulent or making a false statement to avoid, decrease or conceal an obligation to pay money to the federal government; | |
| ● | the federal Health Insurance Portability and Accountability Act of 1996 (“HIPAA”) which imposes criminal and civil liability for executing a scheme to defraud any healthcare benefit program and also created federal criminal laws that prohibit knowingly and willfully falsifying, concealing or covering up a material fact or making any materially false statements in connection with the delivery of or payment for healthcare benefits, items or services, as amended by the Health Information Technology for Economic and Clinical Health Act of 2009 (“HITECH”) which imposes obligations, including mandatory contractual terms, with respect to safeguarding the privacy, security and transmission of individually identifiable health information on entities subject to the law, such as certain healthcare providers, health plans, and healthcare clearinghouses, known as covered entities, and their respective business associates that perform services for them that involve the creation, use, maintenance or disclosure of, individually identifiable health information; |
| -41- |
| ● | the federal physician sunshine requirements under the ACA which requires certain manufacturers of drugs, devices, biologics and medical supplies, with certain exceptions, to report annually to HHS information related to payments and other transfers of value to physicians, other healthcare providers, and teaching hospitals, and ownership and investment interests held by physicians and other healthcare providers and their immediate family members and applicable group purchasing organizations; | |
| ● | analogous state and foreign laws and regulations, such as state anti-kickback and false claims laws, which might apply to sales or marketing arrangements and claims involving healthcare items or services reimbursed by non-governmental third party payors, including private insurers; some state laws which require pharmaceutical companies to comply with the pharmaceutical industry’s voluntary compliance guidelines and the relevant compliance guidance promulgated by the federal government and might require drug manufacturers to report information related to payments and other transfers of value to physicians and other healthcare providers, marketing expenditures or pricing information; and certain state and local laws which require the registration of pharmaceutical sales representatives; and | |
| ● | state and foreign laws govern the privacy and security of health information in specified circumstances, many of which differ from each other in significant ways and often are not pre-empted by HIPAA, thus complicating compliance efforts. |
Efforts to ensure that our business arrangements with third parties will comply with applicable healthcare laws and regulations will involve substantial costs. It is possible that governmental authorities will conclude that our business practices might not comply with current or future statutes, regulations or case law involving applicable fraud and abuse or other healthcare laws and regulations. If our operations are found to be in violation of any of these laws or any other governmental regulations that might apply to us, we might be subject to significant civil, criminal and administrative penalties, damages, fines, imprisonment, disgorgement, exclusion from government funded healthcare programs, such as Medicare and Medicaid, integrity oversight and reporting obligations, and the curtailment or restructuring of our operations. If any physicians or other healthcare providers or entities with whom we expect to do business are found to not be in compliance with applicable laws, they might be subject to criminal, civil or administrative sanctions, including exclusions from government funded healthcare programs.
Our employees might engage in misconduct or other improper activities, including noncompliance with regulatory standards and requirements, which could cause significant liability for us and harm our reputation.
We are exposed to the risk of employee fraud or other misconduct, including intentional failures to comply with FDA regulations or similar regulations of comparable foreign regulatory authorities, provide accurate information to the FDA or comparable foreign regulatory authorities, comply with manufacturing standards we have established, comply with federal and state healthcare fraud and abuse laws and regulations and similar laws and regulations established and enforced by comparable foreign regulatory authorities, report financial information or data accurately or disclose unauthorized activities to us. Employee misconduct could also involve the improper use of information obtained in the course of clinical trials, which could result in regulatory sanctions and serious harm to our reputation. It is not always possible to identify and deter employee misconduct, and the precautions we take to detect and prevent this activity might not be effective in controlling unknown or unmanaged risks or losses or in protecting us from governmental investigations or other actions or lawsuits stemming from a failure to be in compliance with such laws or regulations. If any such actions are instituted against us, and we are not successful in defending ourselves or asserting our rights, those actions could have a significant impact on our business and results of operations, including the imposition of significant civil, criminal and administrative penalties, damages, fines, imprisonment, exclusion from government funded healthcare programs, such as Medicare and Medicaid, and integrity oversight and reporting obligations.
| -42- |
Product liability lawsuits against us could cause us to incur substantial liabilities and to limit commercialization of any products that we might develop.
We face an inherent risk of product liability exposure related to the testing of our lead product candidate or future product candidates in human clinical trials and will face an even greater risk if we commercially sell any products that we might develop. Product liability claims might be brought against us by subjects enrolled in our clinical trials, patients, healthcare providers or others using, administering or selling our intended product. If we cannot successfully defend ourselves against claims that our lead product candidate or product caused injuries, we could incur substantial liabilities. Regardless of merit or eventual outcome, liability claims might result in:
| ● | decreased demand for any product candidates or products that we might develop; | |
| ● | termination of clinical trial sites or entire clinical trial programs; | |
| ● | injury to our reputation and significant negative media attention; | |
| ● | withdrawal of clinical trial participants; | |
| ● | significant costs to defend the related litigation; | |
| ● | substantial monetary awards to trial subjects or patients; | |
| ● | loss of revenue; | |
| ● | diversion of management and scientific resources from our business operations; and | |
| ● | the inability to commercialize any products that we might develop. |
Prior to engaging in clinical trials, we obtain product liability insurance coverage at a level that we believe is customary for similarly situated companies and adequate to provide us with insurance coverage for foreseeable risks; however, we might be unable to obtain such coverage at a reasonable cost, if at all. If we are able to obtain product liability insurance, we might not be able to maintain insurance coverage at a reasonable cost or in an amount adequate to satisfy any liability that might arise and such insurance might not be adequate to cover all liabilities that we might incur. Furthermore, we intend to expand our insurance coverage for products to include the sale of commercial products if we obtain regulatory approval for our lead product candidate in development, but we might be unable to obtain commercially reasonable product liability insurance for any products that receive regulatory approval. Large judgments have been awarded in class action lawsuits based on drugs that had unanticipated side effects. A successful product liability claim or series of claims brought against us, particularly if judgments exceed our insurance coverage, could decrease our cash and adversely affect our business.
Risks Related to our Business Operations
We face substantial competition, which might result in others discovering, developing or commercializing products before or more successfully than we do.
We will face competition from numerous pharmaceutical and biotechnology enterprises, as well as from academic institutions, government agencies and private and public research institutions for our lead product candidate. Our commercial opportunities will be reduced or eliminated if our competitors develop and commercialize products that are safer, more effective, have fewer side effects or are less expensive than any products that we might develop. Competition could result in reduced sales and pricing pressure on our lead product candidate, if approved, which in turn would reduce our ability to generate meaningful revenues and have a negative impact on our results of operations. In addition, significant delays in the development of our lead product candidate could allow our competitors to bring products to market before we do and impair our ability to commercialize our lead product candidate. The biotechnology industry, including the cancer immunotherapy market, is intensely competitive and involves a high degree of risk. We compete with other companies that have far greater experience and financial, research and technical resources than us. Potential competitors in the United States and worldwide are numerous and include pharmaceutical and biotechnology companies, educational institutions and research foundations, many of which have substantially greater capital resources, marketing experience, research and development staffs and facilities than ours. Some of our competitors might develop and commercialize products that compete directly with those incorporating our technology or might introduce products to market earlier than our intended product or on a more cost-effective basis. Our competitors compete with us in recruiting and retaining qualified scientific and management personnel as well as in acquiring technologies complementary to our technology. We might face competition with respect to product efficacy and safety, ease of use and adaptability to various modes of administration, acceptance by physicians, the timing and scope of regulatory approvals, availability of resources, reimbursement coverage, price and patent position, including the potentially dominant patent positions of others. An inability to successfully complete our product development or commercializing our lead product candidate could result in our having limited prospects for establishing market share or generating revenue.
| -43- |
Many of our competitors or potential competitors have significantly greater established presence in the market, financial resources and expertise in research and development, manufacturing, preclinical testing, conducting clinical trials, obtaining regulatory approvals and marketing approved products than we do, and as a result might have a competitive advantage over us. Mergers and acquisitions in the pharmaceutical and biotechnology industries might result in even more resources being concentrated among a smaller number of our competitors. Smaller or early-stage companies might also prove to be significant competitors, particularly through collaborative arrangements with large and established companies. These third parties compete with us in recruiting and retaining qualified scientific and management personnel, establishing clinical trial sites and patient registration for clinical trials, as well as in acquiring technologies and technology licenses complementary to our programs or potentially advantageous to our business.
As a result of these factors, these competitors might obtain regulatory approval of their products before we are able to obtain patent protection or other intellectual property rights, which will limit our ability to develop or commercialize our lead product candidate. Our competitors might also develop drugs that are safer, more effective, more widely used and cheaper than ours, and might also be more successful than us in manufacturing and marketing their products. These appreciable advantages could render our lead product candidate obsolete or non-competitive before we can recover the expenses of development and commercialization.
Our business might be adversely affected by the coronavirus or other pandemics.
The global outbreak of the novel coronavirus (Covid-19) in early 2020 led to disruptions in general economic activities throughout the world as businesses and governments implemented broad actions to mitigate this public health crisis. Although the Covid-19 outbreak has subsided, the extent to which the coronavirus pandemic may reappear and impact the Company’s clinical trial programs and capital raising efforts in the future is uncertain and cannot be predicted.
Significant disruptions of information technology systems, computer system failures or breaches of information and cyber security could adversely affect our business.
We rely to a large extent upon sophisticated information technology systems to operate our business. In the ordinary course of business, we collect, store and transmit large amounts of confidential information (including, but not limited to, personal information and intellectual property). The size and complexity of our information technology and information security systems, and those of our third party vendors with whom we might contract, make such systems potentially vulnerable to service interruptions or to security breaches from inadvertent or intentional actions by our employees or vendors, or from malicious attacks by third parties. Such attacks are of ever-increasing levels of sophistication and are made by groups and individuals with a wide range of motives (including, but not limited to, industrial espionage and market manipulation) and expertise. While we intend to invest in the protection of data and information technology, there can be no assurance that our efforts will prevent service interruptions or security breaches.
Our internal computer systems, and those of our CROs, our CMOs, and other business vendors on which we might rely, are vulnerable to damage from computer viruses, unauthorized access, natural disasters, fire, terrorism, war and telecommunication and electrical failures. We exercise little or no control over these third parties, which increases our vulnerability to problems with their systems. If such an event were to occur and cause interruptions in our operations, it could result in a material disruption of our drug development programs. Any interruption or breach in our systems could adversely affect our business operations and/or result in the loss of critical or sensitive confidential information or intellectual property, and could result in financial, legal, business and reputational harm to us or allow third parties to gain material, inside information that they use to trade in our securities. For example, the loss of clinical trial data from completed or ongoing clinical trials could result in delays in our regulatory approval efforts and significantly increase our costs to recover or reproduce the data. To the extent that any disruption or security breach results in a loss of or damage to our data or applications, or misappropriation or disclosure of confidential or proprietary information, we could incur liability, the further development of our lead and future product candidates could be delayed and our business could be otherwise adversely affected.
| -44- |
We might need to grow the size of our organization in the future, and we might experience difficulties in managing this growth.
As of March 14, 2025, we had two officer/employees, our Chief Executive Officer and our Chief Financial Officer, and one consultant, our Chief Medical Officer. The Company relies to a significant extent on outside consultants and advisors with various technical skills and expertise that the Company can draw on as necessary to conduct its research and development and clinical trial programs. We might need to grow the size of our organization in order to support our continued development and potential commercialization of our lead product candidate. As our development and commercialization plans and strategies continue to develop, our need for additional managerial, operational, manufacturing, sales, marketing, financial and other resources might increase. Our management, personnel and systems currently in place might not be adequate to support this future growth. Future growth would impose significant added responsibilities on members of management, including:
| ● | managing our clinical trials effectively; | |
| ● | identifying, recruiting, maintaining, motivating and integrating additional employees; | |
| ● | managing our internal development efforts effectively while complying with our contractual obligations to licensors, licensees, contractors and other third parties; | |
| ● | improving our managerial, development, operational, information technology, and finance systems; and | |
| ● | expanding our facilities. |
If our operations expand, we will likely also need to manage additional relationships with various strategic partners, suppliers and other third parties. Our future financial performance and our ability to commercialize our lead product candidate and to compete effectively will depend, in part, on our ability to manage any future growth effectively, as well as our ability to develop a sales and marketing force when appropriate for our company. To that end, we must be able to manage our development efforts and preclinical studies and clinical trials effectively and hire, train and integrate additional management, research and development, manufacturing, administrative and sales and marketing personnel. The failure to accomplish any of these tasks could prevent us from successfully growing our company.
Inadequate funding for the FDA, the SEC and other government agencies could hinder their ability to hire and retain key leadership and other personnel, prevent new products and services from being developed or commercialized in a timely manner or otherwise prevent those agencies from performing normal business functions on which the operation of our business might rely, which could negatively impact our business.
The ability of the FDA to review and approve new products can be affected by a variety of factors, including government budget and funding levels, ability to hire and retain key personnel and accept the payment of user fees, and statutory, regulatory, and policy changes. Average review times at the agency have fluctuated in recent years as a result. In addition, government funding of the SEC and other government agencies on which our operations might rely, including those that fund research and development activities is subject to the political process, which is inherently fluid and unpredictable.
Disruptions at the FDA and other agencies might also slow the time necessary for new drugs to be reviewed and/or approved by necessary government agencies, which would adversely affect our business. For example, over the last several years, the U.S. government has shut down several times for various periods of time, and certain regulatory agencies, such as the FDA and the SEC, have had to furlough critical FDA, SEC and other government employees and stop critical activities. If a prolonged government shutdown occurs, it could significantly impact the ability of the FDA to timely review and process our regulatory submissions, which could have a material adverse effect on our business. Further, in our operations as a public company, future government shutdowns could impact our ability to access the public markets and obtain necessary capital in order to properly capitalize and continue our operations.
| -45- |
Unstable market and economic conditions and adverse developments with respect to financial institutions and associated liquidity risk may have serious adverse consequences on our business, financial condition and stock price.
The global credit and financial markets have recently experienced extreme volatility and disruptions, including severely diminished liquidity and credit availability, declines in consumer confidence, declines in economic growth, inflationary pressures and interest rate changes, increases in unemployment rates and uncertainty about economic stability. The financial markets and the global economy may also be adversely affected by the current or anticipated impact of military conflict, including the conflict between Russia and Ukraine, between Israel and Gaza, terrorism or other geopolitical events. Sanctions imposed by the United States and other countries in response to such conflicts, including the one in Ukraine, may also adversely impact the financial markets and the global economy, and any economic countermeasures by the affected countries or others could exacerbate market and economic instability. Future adverse developments with respect to financial institutions or the broader financial services industry may lead to market-wide liquidity shortages, impair the ability of companies to access near-term working capital needs, and create additional market and economic uncertainty. There can be no assurance that future credit and financial market instability and a deterioration in confidence in economic conditions will not occur. Our general business strategy may be adversely affected by any such economic downturn, liquidity shortages, volatile business environment or continued unpredictable and unstable market conditions. If the equity markets deteriorate, or if adverse developments are experienced by financial institutions, it may cause short-term liquidity risk and also make any necessary equity financing more difficult, more costly and more dilutive. Failure to secure any necessary financing in a timely manner and on favorable terms could have a material adverse effect on our business plans and stock price and could require us to delay or abandon clinical development plans. In addition, there is a risk that one or more of our current service providers, financial institutions, manufacturers and other partners may be adversely affected by the foregoing risks, which could directly affect our ability to conduct our business plans on schedule and on budget.
Risks Related to Owning our Securities
We are a “smaller reporting company” and we have elected to comply with certain reduced reporting and disclosure requirements which could make its common stock less attractive to investors.
We are a “smaller reporting company,” as defined in the Regulation S-K of the Securities Act of 1933, as amended (the “Securities Act”), which allows us to take advantage of certain exemptions from various reporting requirements that are applicable to other public companies that are not smaller reporting companies, including (1) not being required to comply with the auditor attestation requirements of Section 404 of the Sarbanes-Oxley Act of 2002, and (2) reduced disclosure obligations regarding executive compensation in our periodic reports and proxy statements. In addition, as an emerging growth company, we are only required to provide two years of audited financial statements in this document. As a result of these reduced reporting and disclosure requirements our financial statements might not be comparable to SEC registrants not classified as emerging growth companies.
We cannot predict if investors will find our common stock less attractive because we might rely on these exemptions. If some investors find our common stock less attractive as a result, there might be a less active trading market for our common stock and our stock price might be more volatile.
Our independent registered public accounting firm is not required to formally attest to the effectiveness of our internal control over financial reporting until we are no longer a “smaller reporting company”. We cannot assure you that there will not be material weaknesses or significant deficiencies in our internal controls in the future.
Investors might find our common stock less attractive as a result of our election to utilize these exemptions, which could result in a less active trading market for our common stock and/or the market price of our common stock might be more volatile.
The publicly-traded warrants that we issued in our November 2020 public offering are speculative in nature.
The warrants issued in our November 2020 public offering do not confer any rights of common stock ownership on their holders, such as voting rights or the right to receive dividends, but rather merely represent the right to acquire shares of common stock at a fixed price. Specifically, holders of the warrants may exercise their right to acquire the common stock and pay an effective exercise price of $57.00 per share, which is substantially in excess of the current market price of the Company’s common stock. Furthermore, each warrant will expire five years from the original issuance date, which is November 20, 2025. In the event our common stock price does not exceed the exercise price of the warrants during the period when the warrants are exercisable, the warrants may not have any value.
| -46- |
Holders of the warrants have no rights as a common stockholder until they acquire our common stock.
Until the acquisition of shares of our common stock upon exercise of the warrants, a holder has no rights with respect to shares of our common stock issuable upon exercise of the warrant. Upon exercise of a warrant, a holder will be entitled to exercise the rights of a common stockholder as to the security exercised only as to matters for which the record date occurs after the exercise.
There is a limited market for the warrants to purchase shares of our common stock.
Although the warrants are currently trading on The Nasdaq Capital Market, there can be no assurance that an active trading market for the warrants will develop. Without an active trading market, the liquidity of the warrants will continue to be limited.
Provisions of certain warrants could discourage a change-in control transaction involving a third party.
Some of our warrants contain provisions that could make it more difficult or expensive for a third party to make an investment in us acquire us in a change-in-control transaction. Under certain transactions constituting a “fundamental transaction”, the Company could be required to redeem the warrants for a cash payment calculated pursuant to the Black-Scholes option-pricing model. These and other provisions of the warrants could prevent or deter a third party from acquiring us or investing in us in a change-in control transaction, even where the acquisition could be beneficial to you.
July 20, 2023 sale of common stock and warrants.
On July 20, 2023, we sold 583,334 shares of common stock at a price of $6.00 per share to an institutional investor and raised gross proceeds of approximately $3,500,000. As part of this financing, the Company sold warrants to the institutional investor to purchase 583,334 shares of common stock. The common warrants had an initial exercise price of $6.00 per share, were immediately exercisable upon issuance, and expire five years thereafter on July 20, 2028. The Company also issued warrants to the placement agent to purchase 35,000 shares of common stock at an exercise price of $6.60 per share and expiring on July 20, 2028.
The exercise prices of the warrants issued to the institutional investor and to the placement agent are subject to customary adjustments for stock splits, stock dividends, stock combinations, reclassifications, reorganizations, or similar events affecting the Company’s common stock. In addition, the warrants issued to the institutional investor contain a “fundamental transaction” provision whereby in the event of a fundamental transaction (including a sale or transfer of assets or ownership of the Company as defined in the warrant agreement) within the Company’s control, the holder of the unexercised common stock warrants would be entitled to receive, in exchange for extinguishment of the warrants, cash consideration equal to a Black-Scholes valuation, as defined in the warrant agreement. If such fundamental transaction is not within the Company’s control, the warrant holder would only be entitled to receive the same form of consideration (and in the same proportion) as the holders of the Company’s common stock.
Accordingly, in the event of a change in control of the Company or a sale or transfer of all or substantially all of the Company’s assets, as defined in the warrants, to the extent that the warrants issued to the institutional investor are outstanding at the effective date that such a transaction is closed, this “fundamental transaction” provision would entitle the holder to substantial cash consideration, thus reducing the amounts to be retained by the Company or potentially distributable to the Company’s stockholders.
February 13, 2025 sale of common stock and warrants.
On February 13, 2025, we sold 434,784 shares of common stock at a price of $2.415 per share to two institutional investors and raised gross proceeds of approximately $1,050,000. As part of this financing, the Company sold warrants to the institutional investors to purchase 434,784 shares of common stock. The common warrants had an initial exercise price of $2.29 per share, were immediately exercisable upon issuance, and expire five years thereafter on February 13, 2030. The Company also issued warrants to the placement agent to purchase 32,609 shares of common stock at an exercise price of $3.0188 per share and expiring on February 13, 2030.
| -47- |
The exercise prices of the warrants issued to the institutional investors and to the placement agent are subject to customary adjustments for stock splits, stock dividends, stock combinations, reclassifications, reorganizations, or similar events affecting the Company’s common stock. In addition, the warrants issued to the institutional investors contain a “fundamental transaction” provision whereby in the event of a fundamental transaction (including a sale or transfer of assets or ownership of the Company as defined in the warrant agreement) within the Company’s control, the holders of the unexercised common stock warrants would be entitled to receive, in exchange for extinguishment of the warrants, cash consideration equal to a Black-Scholes valuation, as defined in the warrant agreement. If such fundamental transaction is not within the Company’s control, the warrant holders would only be entitled to receive the same form of consideration (and in the same proportion) as the holders of the Company’s common stock.
Accordingly, in the event of a change in control of the Company or a sale or transfer of all or substantially all of the Company’s assets, as defined in the warrants, to the extent that the warrants issued to the institutional investors are outstanding at the effective date that such a transaction is closed, this “fundamental transaction” provision would entitle the holders to substantial cash consideration, thus reducing the amounts to be retained by the Company or potentially distributable to the Company’s stockholders.
Our management has broad discretion over the use of the proceeds from any stock offerings we may conduct in the future and we may apply it to uses that do not improve our operating results or the value of our common stock.
Our management will have broad discretion in the application of the net proceeds from any stock offerings, and investors will be relying solely on the judgment of our management regarding the application of these proceeds. Although we expect to use the net proceeds from an offering for working capital and general corporate purposes, including the ongoing clinical development of our lead compound LB-100, we have not allocated these net proceeds for specific purposes. Investors will not have the opportunity, as part of their investment decision, to assess whether the proceeds are being used appropriately. Our use of the proceeds may not improve our business prospects or increase the value of our common stock.
As part of the Company’s ongoing process of evaluating various alternatives to obtain the capital required to fund its operations and maintain its listing on Nasdaq, management may decide to consider a wide variety of strategic alternatives, and there can be no assurances that any such transaction, if implemented, would enhance stockholder value, and could be highly dilutive to existing stockholders.
The Company is evaluating various alternatives to obtain the capital required to fund its operations and maintain its listing on Nasdaq, including merger or acquisition opportunities (including reverse mergers) and funding transactions involving a change in control. There can be no assurances that the evaluation process will result in the identification of an appropriate transaction, the negotiation and execution of a definitive agreement to effect such a transaction, or that any such transaction will ultimately be approved by the Company’s stockholders and then be consummated. Depending on various factors, many of which are outside the control of the Company, our failure to enter into and consummate a strategic transaction could have a material adverse effect on our ability to continue to operate and finance our business, and on the market price of our common stock. Even if such a strategic transaction is consummated, there can be no assurances that it will enhance stockholder value, and it may result in substantial dilution to existing stockholders. Any potential transaction would be dependent on a number of factors that may be outside of our control, including, among other things, market conditions, industry trends, the interest of third parties in a potential transaction with the Company, and the availability of appropriate financing for such a transaction. If we are unable to raise the required capital to fund our operations, or to enter into a strategic transaction in the near future, we may need to curtail or cease operations, which could result in a total loss of stockholders’ investment.
The price of our common stock or warrants might fluctuate substantially.
You should consider an investment in our common stock and warrants to be risky. Some factors that might cause the market price of our common stock or warrants to fluctuate, in addition to the other risks mentioned in this “Risk Factors,” are:
| ● | sale of our common stock by our stockholders, executives, and directors and our stockholders; |
| -48- |
| ● | volatility and limitations in trading volumes of our shares of common stock; | |
| ● | our ability to obtain financings to conduct and complete research and development activities including, but not limited to, our clinical trials, and other business activities; | |
| ● | the timing and success of introductions of new products by us or our competitors or any other change in the competitive dynamics of our industry, including consolidation among competitors, customers or strategic partners; | |
| ● | network outages or security breaches; | |
| ● | our ability to secure resources and the necessary personnel to conduct clinical trials on our desired schedule; | |
| ● | commencement, enrollment or results of our clinical trials for our lead product candidate or any future clinical trials we might conduct; | |
| ● | changes in the development status of our lead product candidate; | |
| ● | any delays or adverse developments or perceived adverse developments with respect to the FDA’s review of our planned preclinical and clinical trials; | |
| ● | any delay in our submission for studies or product approvals or adverse regulatory decisions, including failure to receive regulatory approval for our lead product candidate; | |
| ● | unanticipated safety concerns related to the use of our lead product candidate; | |
| ● | failures to meet external expectations or management guidance; | |
| ● | changes in our capital structure or dividend policy, future issuances of securities, sales of large blocks of common stock by our stockholders; | |
| ● | our cash position; | |
| ● | announcements and events surrounding financing efforts, including debt and equity securities; | |
| ● | our inability to enter into new markets or develop new products; | |
| ● | reputational issues; | |
| ● | competition from existing technologies and products or new technologies and products that might emerge; | |
| ● | announcements of acquisitions, partnerships, collaborations, joint ventures, new products, capital commitments, or other events by us or our competitors; | |
| ● | changes in general economic, political and market conditions in or any of the regions in which we conduct our business; | |
| ● | changes in industry conditions or perceptions; | |
| ● | changes in valuations of similar companies or groups of companies; | |
| ● | analyst research reports, recommendation and changes in recommendations, price targets, and withdrawals of coverage; |
| -49- |
| ● | departures and additions of key personnel; | |
| ● | disputes and litigations related to intellectual properties, proprietary rights, and contractual obligations; | |
| ● | changes in applicable laws, rules, regulations, or accounting practices and other dynamics; and | |
| ● | other events or factors, many of which might be out of our control. |
In addition, if the market for stocks in our industry or industries related to our industry, or the stock market in general, experiences a loss of investor confidence, the trading price of our common stock could decline for reasons unrelated to our business, financial condition and results of operations. If any of the foregoing occurs, it could cause our stock price to fall and might expose us to lawsuits that, even if unsuccessful, could be costly to defend and a distraction to management.
Risks Related to the Company’s Common Stock
Nasdaq Compliance.
The Company’s common stock and the warrants are traded on The Nasdaq Capital Market under the symbols “LIXT” and “LIXTW”, respectively.
On June 2, 2023, the Company effected a 1-for-10 reverse split of its outstanding shares of common stock in order to remain in compliance with the $1.00 minimum closing bid price requirement of Nasdaq. However, there can be no assurances that the Company will be able to remain in compliance with the $1.00 minimum closing bid price requirement of Nasdaq over time. In addition, Nasdaq has other continued listing requirements, one of which is maintaining a minimum net stockholders’ equity of $2,500,000.
On August 23, 2024, the Company received a letter from the Listing Qualifications Department (the “Staff”) of the Nasdaq Stock Market LLC (“Nasdaq”) on August 19, 2024 indicating that the Company was not in compliance with the minimum stockholders’ equity requirement of $2,500,000 for continued listing on the Nasdaq Capital Market under Listing Rule 5550(b) (the “Stockholders’ Equity Requirement”).
On October 3, 2024, the Company submitted a plan to the Staff to regain compliance with the Stockholders’ Equity Requirement, which outlined the Company’s proposed initiatives to regain compliance by raising equity capital through various registered equity offerings.
On October 21, 2024, the Staff provided notice (the “Notice”) to the Company that it had granted an extension through February 18, 2025 to regain compliance with the Stockholders’ Equity Requirement, which required that the Company complete its capital raising initiatives and evidence compliance with the Stockholders’ Equity Requirement through filing a Current Report on Form 8-K with the Securities and Exchange Commission (the “SEC”) providing certain required information.
As of February 18, 2025, the Company had not gained compliance with the Stockholders’ Equity Requirement. Accordingly, on February 19, 2025, the Company received a Staff determination letter from the Staff stating that the Company did not meet the terms of the extension because it did not complete its proposed financing initiatives to regain compliance.
The Company timely filed an appeal and requested a Hearing before a Nasdaq Hearings Panel (the “Panel”), which has been granted. The Hearing request automatically stayed Nasdaq’s delisting of the Company’s common shares and warrants pending the Panel’s decision. Pursuant to the Nasdaq Listing Rules, the Panel has the discretion to grant the Company an additional extension through no later than August 18, 2025. At the upcoming hearing, the Company will present its plan for regaining and sustaining compliance with the Stockholders’ Equity Requirement for continued listing. However, there can be no assurances that the Hearings Panel will grant the Company an extension of time to regain compliance, or that the Company will be able to regain compliance during any extension period. During the appeal process the Company’s common shares and warrants will continue to trade on The Nasdaq Capital Market.
| -50- |
The Company intends to take reasonable measures available to regain compliance under Nasdaq’s listing rules and to remain listed on Nasdaq. However, there can be no assurances that the Company will ultimately regain compliance with the Stockholders’ Equity Rule, or be able to maintain compliance with all other applicable requirements for continued listing on Nasdaq. If the Company does not regain compliance with Nasdaq’s continued listing requirements within the time period permitted by Nasdaq, then the Company’s securities will be delisted from Nasdaq.
If the Company were to be delisted from Nasdaq, its common stock and warrants may be eligible for trading on an over-the-counter market. If the Company is not able to obtain a listing on another stock exchange or quotation service for its common stock and warrants, it may be extremely difficult or impossible for stockholders to sell their shares of common stock and warrants. Moreover, if the Company is delisted from Nasdaq, but obtains a substitute listing for its common stock and warrants, it will likely be on a market with less liquidity, and therefore experience potentially more price volatility than experienced on Nasdaq. Stockholders may not be able to sell their shares of common stock and warrants on any such substitute market in the quantities, at the times, or at the prices that could potentially be available on a more liquid trading market. As a result of these factors, if the Company’s common stock is delisted from Nasdaq, the value and liquidity of the Company’s common stock and warrants would likely be significantly adversely affected. A delisting of the Company’s common stock from Nasdaq could also adversely affect the Company’s ability to obtain financing for its operations and/or could result in a loss of confidence by investors, employees and/or business partners.
A sale or perceived sale of a substantial number of shares of our common stock might cause the price of our common stock to decline.
If our stockholders sell substantial amounts of our common stock in the public market, the market price of our common stock could fall. Moreover, the perceived risk of this potential dilution could cause stockholders to attempt to sell their shares and investors to short our common stock. These sales also might make it more difficult for us to sell equity or equity-related securities in the future at a time and price that we deem reasonable or appropriate.
Market and economic conditions might negatively impact our business, financial condition and share price.
Concerns over medical epidemics, energy costs, geopolitical issues, the U.S. mortgage market and a deteriorating real estate market, unstable global credit markets and financial conditions, and volatile oil prices have led to periods of significant economic instability, diminished liquidity and credit availability, declines in consumer confidence and discretionary spending, diminished expectations for the global economy and expectations of slower global economic growth, increased unemployment rates, and increased credit defaults in recent years. Our general business strategy might be adversely affected by any such economic downturns (including the impact related to the recent COVID-19 pandemic), volatile business environments and continued unstable or unpredictable economic and market conditions. If these conditions continue to deteriorate or do not improve, it might make any necessary debt or equity financing more difficult to complete, more costly, and more dilutive. Failure to secure any necessary financing in a timely manner and on favorable terms could have a material adverse effect on our growth strategy, financial performance, and share price and could require us to delay or abandon development or commercialization plans.
If securities or industry analysts do not publish research or reports, or publish unfavorable research or reports about our business, our stock price and trading volume might decline.
The trading market for our common stock will rely in part on the research and reports that industry or financial analysts publish about us, our business, our markets and our competitors. We do not control these analysts. If securities analysts do not cover our common stock, the lack of research coverage might adversely affect the market price of our common stock. Furthermore, if one or more of the analysts who do cover us downgrade our stock or if those analysts issue other unfavorable commentary about us or our business, our stock price would likely decline. If one or more of these analysts cease coverage of us or fails to regularly publish reports on us, we could lose visibility in the market and interest in our stock could decrease, which in turn could cause our stock price or trading volume to decline and might also impair our ability to expand our business with existing customers and attract new customers.
| -51- |
Future sales and issuances of our common stock could result in additional dilution of the percentage ownership of our stockholders and could cause our share price to fall.
In the future, we will need to issue additional authorized but previously unissued equity securities, resulting in the dilution of the ownership interests of our stockholders. We may also issue additional common stock, warrants or other securities that are convertible into or exercisable for common stock in connection with future mergers or acquisitions, future sales of securities for capital raising purposes, or for other business purposes, in one or more transactions at prices and in a manner that we determine from time to time. The future issuance of any such additional shares of common stock may create downward pressure on the trading price of the common stock. There can be no assurances that we will not be required to issue additional shares, warrants or other convertible securities in the future in conjunction with any capital raising efforts, and the new investors could gain rights superior to our existing stockholders in any such transactions.
We do not intend to pay cash dividends on our shares of common stock so any returns will be limited to the value of our shares.
We currently anticipate that we will retain future earnings for the development, operation and expansion of our business and do not anticipate declaring or paying any cash dividends for the foreseeable future. Any return to stockholders will therefore be limited to the increase, if any, of our share price.
We might be at risk of securities class action litigation.
We might be at risk of securities class action litigation. In the past, biotechnology and pharmaceutical companies have experienced significant stock price volatility, particularly when associated with binary events such as clinical trials and product approvals. If we face such litigation, it could result in substantial costs and a diversion of management’s attention and resources, which could harm our business and results in a decline in the market price of our common stock.
Our Certificate of Incorporation and our Amended and Restated Bylaws, and Delaware law might have anti-takeover effects that could discourage, delay or prevent a change in control, which might cause our stock price to decline.
Our Certificate of Incorporation and our Amended and Restated Bylaws, and Delaware law could make it more difficult for a third party to acquire us, even if closing such a transaction would be beneficial to our stockholders. We are authorized to issue up to 10,000,000 shares of preferred stock. This preferred stock might be issued in one or more series, the terms of which might be determined at the time of issuance by our Board of Directors without further action by stockholders. The terms of any series of preferred stock might include voting rights (including the right to vote as a series on particular matters), preferences as to dividend, liquidation, conversion and redemption rights and sinking fund provisions. We have designated 350,000 shares of preferred stock as Series A Convertible Preferred Stock, all of which are issued and outstanding. The issuance of any preferred stock could materially adversely affect the rights of the holders of our common stock, and therefore, reduce the value of our common stock. In particular, specific rights granted to future holders of preferred stock could be used to restrict our ability to merge with, or sell our assets to, a third party and thereby preserve control by the present management.
Provisions of our Certificate of Incorporation and our Amended and Restated Bylaws and Delaware law also could have the effect of discouraging potential acquisition proposals or making a tender offer or delaying or preventing a change in control, including changes a stockholder might consider favorable. Such provisions might also prevent or frustrate attempts by our stockholders to replace or remove our management. In particular, the certificate of incorporation and bylaws and Delaware law, as applicable, among other things:
| ● | provide the Board of Directors with the ability to alter the bylaws without stockholder approval; | |
| ● | place limitations on the removal of directors; | |
| ● | establishing advance notice requirements for nominations for election to the Board of Directors or for proposing matters that can be acted upon at stockholder meetings; and | |
| ● | provide that vacancies on the Board of Directors might be filled by a majority of directors in office, although less than a quorum. |
| -52- |
Financial reporting obligations of being a public company in the United States are expensive and time-consuming, and our management will be required to devote substantial time to compliance matters.
As a publicly traded company we incur significant additional legal, accounting and other expenses. The obligations of being a public company in the United States require significant expenditures and will place significant demands on our management and other personnel, including costs resulting from public company reporting obligations under the Exchange Act and the rules and regulations regarding corporate governance practices, including those under the Sarbanes-Oxley Act, the Dodd-Frank Wall Street Reform and Consumer Protection Act, and the listing requirements of the stock exchange on which our securities are listed. These rules require the establishment and maintenance of effective disclosure and financial controls and procedures, internal control over financial reporting and changes in corporate governance practices, among many other complex rules that are often difficult to implement, monitor and maintain compliance with. Moreover, despite recent reforms made possible by the JOBS Act, the reporting requirements, rules, and regulations will make some activities more time-consuming and costly, particularly after we are no longer an “emerging growth company”. In addition, we expect these rules and regulations to make it more difficult and more expensive for us to obtain director and officer liability insurance. Our management and other personnel will need to devote a substantial amount of time to ensure that we comply with all of these requirements and to keep pace with new regulations, otherwise we might fall out of compliance and risk becoming subject to litigation or being delisted, among other potential problems.
If we fail to comply with the rules under Sarbanes-Oxley related to accounting controls and procedures in the future, or, if we discover material weaknesses and other deficiencies in our internal control and accounting procedures, our stock price could decline significantly and raising capital could be more difficult.
Section 404 of Sarbanes-Oxley requires annual management assessments of the effectiveness of our internal control over financial reporting. If we fail to comply with the rules under Sarbanes-Oxley related to disclosure controls and procedures in the future, or, if we discover material weaknesses and other deficiencies in our internal control and accounting procedures, our stock price could decline significantly and raising capital could be more difficult. If material weaknesses or significant deficiencies are discovered or if we otherwise fail to achieve and maintain the adequacy of our internal control, we might not be able to ensure that we can conclude on an ongoing basis that we have effective internal controls over financial reporting in accordance with Section 404 of Sarbanes-Oxley. Moreover, effective internal controls are necessary for us to produce reliable financial reports and are important to helping prevent financial fraud. If we cannot provide reliable financial reports or prevent fraud, our business and operating results could be harmed, investors could lose confidence in our reported financial information, and the trading price of our common stock could drop significantly.
ITEM 1B. UNRESOLVED STAFF COMMENTS
None.
ITEM 1C. CYBERSECURITY
| -53- |
Following these risk assessments, we intend to take any steps necessary to redesign, implement, and maintain reasonable safeguards to minimize identified risks; to reasonably address any identified gaps in existing safeguards; and to regularly monitor the effectiveness of our safeguards. Primary responsibility for assessing, monitoring and managing our cybersecurity risks rests with an IT consultant, who reports to our Chief Executive Officer and Chief Financial Officer, to manage the risk assessment and mitigation process. As part of our overall risk management system, we monitor and periodically evaluate our safeguards.
We
may engage consultants, or other
To
date, we have
ITEM 2. PROPERTIES
None.
ITEM 3. LEGAL PROCEEDINGS
The Company may be subject to legal claims and actions from time to time as part of its business activities. As of December 31, 2024, the Company was not subject to any threatened or pending lawsuits, legal claims or legal proceedings.
ITEM 4. MINE SAFETY DISCLOSURES
Not applicable.
| -54- |
PART II
ITEM 5. MARKET FOR REGISTRANT’S COMMON EQUITY, RELATED STOCKHOLDER MATTERS AND ISSUER PURCHASES OF EQUITY SECURITIES
The Company’s common stock and warrants have traded on The Nasdaq Capital Market under the symbols “LIXT” and “LIXTW”, respectively, since November 25, 2020. The stock market in general has experienced significant price fluctuations in the past few years. In some cases, these fluctuations have been unrelated to the operating performance of the affected companies. Many companies have experienced dramatic volatility in the market prices of their common stock. The Company believes that a number of factors, both within and outside its control, could cause the price of the Company’s common stock to fluctuate, perhaps substantially.
The following table sets forth the range of reported closing prices of the Company’s common stock during the periods presented, and have been retroactively adjusted for all periods presented to reflect the 1-for-10 reverse split of the Company’s outstanding shares of common stock effected on June 2, 2023. Such quotations reflect prices between dealers in securities and do not include any retail mark-up, markdown, or commissions, and may not necessarily represent actual transactions.
| Low | High | |||||||
| Year Ended December 31, 2023 | ||||||||
| First Quarter | $ | 5.10 | $ | 18.90 | ||||
| Second Quarter | $ | 4.75 | $ | 9.20 | ||||
| Third Quarter | $ | 1.75 | $ | 7.53 | ||||
| Fourth Quarter | $ | 1.92 | $ | 3.30 | ||||
| Low | High | |||||||
| Year Ended December 31, 2024 | ||||||||
| First Quarter | $ | 1.73 | $ | 3.52 | ||||
| Second Quarter | $ | 2.11 | $ | 3.70 | ||||
| Third Quarter | $ | 1.65 | $ | 2.53 | ||||
| Fourth Quarter | $ | 1.31 | $ | 2.38 | ||||
Holders
As of December 31, 2024, the Company had 35 stockholders of record holding 2,249,290 shares of the Company’s common stock outstanding, including 2,117,561 shares of common stock held by an indeterminate number of beneficial owners of securities whose shares are held in the names of various depository accounts, brokerage firms and clearing agencies.
Dividends
The Company’s dividend policy is determined by its Board of Directors and will depend upon a number of factors, including the Company’s financial condition and performance, its cash needs and expansion plans, income tax consequences, and the restrictions that applicable laws and any credit or other contractual arrangements may then impose. The Company has not paid any cash dividends on its common stock to date and at the current time the Company does not anticipate paying a cash dividend on its common stock in the foreseeable future.
| -55- |
Securities Authorized For Issuance Under Equity Incentive Plans
Set forth in the table below is information regarding awards made through compensation plans or arrangements through December 31, 2024, the most recently completed fiscal year.
| Plan Category | Number
of securities to be issued upon exercise of outstanding options, warrants and rights |
Weighted
average price of outstanding options, warrants and rights |
Number
of securities remaining available for future issuance under compensation plans (excluding securities reflected in column 1) |
|||||||||
| (1) | (2) | (3) | ||||||||||
| Equity Compensation Plans Approved by Security Holders | 567,815 | (1) | $ | 9.858 | 182,185 | (2) | ||||||
| Equity Compensation Plans Not Approved by Security Holders | N/A | $ | N/A | N/A | ||||||||
| (1) | Does not include 45,417 shares issuable that were not issued pursuant to a plan. |
| (2) | The 182,185 shares that remain available are pursuant to the Company’s 2020 Stock Incentive Plan, which was adopted on July 14, 2020 and amended on October 7, 2022 and on November 27, 2023 (see “ITEM 11. EXECUTIVE COMPENSATION”). |
ITEM 6. RESERVED
ITEM 7. MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS
You should read the following discussion and analysis of our financial condition and results of operations together with and our consolidated financial statements and the related notes appearing elsewhere in this Annual Report on Form 10-K. In addition to historical information, this discussion and analysis contains forward-looking statements that involve risks, uncertainties and assumptions. Our actual results may differ materially from those discussed below. Factors that could cause or contribute to such differences include, but are not limited to, those identified below, and those discussed in the section titled “Risk Factors” included elsewhere in this Annual Report on Form 10-K.
Overview
The Company is a clinical-stage biopharmaceutical company focused on identifying new targets for cancer drug development and developing and commercializing cancer therapies. The Company’s corporate office is located in Pasadena, California.
The Company’s product pipeline is primarily focused on inhibitors of protein phosphatase 2A, which is used to enhance cytotoxic agents, radiation, immune checkpoint blockers and other cancer therapies. The Company believes that inhibitors of protein phosphatases have significant therapeutic potential for a broad range of cancers. The Company is focusing on the clinical development of a specific protein phosphatase inhibitor, referred to as LB-100, which has been shown to have clinical anti-cancer activity.
The Company’s activities are subject to significant risks and uncertainties, including the need for additional capital. The Company has not yet commenced any revenue-generating operations, does not have positive cash flows from operations, relies on stock-based compensation for a substantial portion of employee and consultant compensation, and is dependent on periodic access to equity capital to fund its operating requirements.
Recent Significant Developments
Issuance of News Releases
February 25, 2025 - :
The Company announced that it had added the Robert H. Lurie Comprehensive Cancer Center (Lurie Cancer Center) of Northwestern University as a second site in a clinical trial combining the Company’s proprietary compound LB-100 with GSK’s dostarlimab to treat ovarian clear cell cancer.
| -56- |
March 10, 2025 –
The Company announced online publication of new pre-clinical data in BioXriv and International Journal of Pharmaceutics demonstrating how the Company’s lead clinical compound, LB-100, is converted into its active form, endothall, a protein phosphatase (PP2A) inhibitor that has been found to be effective in cancer treatment in combination with immunotherapy.
As published in BioXriv, scientists at the Netherlands Cancer Institute have discovered an enzyme that mediates the conversion of LB-100 into the active metabolite endothall. Accordingly, this protein represents a potential biomarker to identify patients who are most likely to respond to LB100. The biomarker discovery study was performed in the laboratories of Professor Rene Bernards, group leader at the Netherlands Cancer Institute and LIXTE board member.
As published in the International Journal of Pharmaceutics, Dr. Hans Rollema and colleagues, medicinal chemists and biochemists at BioPharmaWorks LLC, a consultant to LIXTE, studied how LB-100 can spontaneously convert into the active metabolite endothall by hydrolysis. Their data indicate that this conversion is slow under physiological conditions. The enzymatic conversion of LB-100 identified by the Bernards laboratory expedites the activation of LB-100 inside the cell.
Other Significant Developments:
Effective March 11, 2025, the Company entered into Amendment No. 1 to the Collaboration Agreement between the Company and GEIS that relieved the Company of the financial obligation to support the randomized Phase 2 portion of the clinical trial contemplated in the Collaboration Agreement of approximately $3,095,000, as more fully described below at Principal Commitments – Clinical Trial Agreements - GEIS.
Going Concern
For the year ended December 31, 2024, the Company recorded a net loss of $3,585,965 and used cash in operations of $3,164,536. At December 31, 2024, the Company had cash of $1,038,952 available to fund its operations. Subsequently, the Company completed a securities offering that generated gross proceeds of $1,050,003 during February 2025 before deducting the placement agent’s fees and related offering expenses.
Because the Company is currently engaged in various early-stage clinical trials, it is expected that it will take a significant amount of time and resources to develop any product or intellectual property capable of generating sustainable revenues. Accordingly, the Company’s business is unlikely to generate any sustainable operating revenues in the next several years and may never do so. Even if the Company is able to generate revenues through licensing its technology, product sales or other commercial activities, there can be no assurance that the Company will be able to achieve and maintain positive earnings and operating cash flows. At March 14, 2025, the Company’s remaining financial contractual commitments pursuant to clinical trial agreements and clinical trial monitoring agreements not yet incurred aggregated approximately $526,000, which are currently scheduled to be incurred through approximately December 31, 2027.
The Company’s consolidated financial statements have been presented on the basis that it will continue as a going concern, which contemplates the realization of assets and satisfaction of liabilities in the normal course of business. The consolidated financial statements also do not reflect any adjustments relating to the recoverability of assets and liabilities that might be necessary if the Company is unable to continue as a going concern. The Company has no recurring source of revenues and has experienced negative operating cash flows since inception. The Company has financed its working capital requirements through the recurring sale of its equity securities.
Based on the foregoing, management has concluded that there is substantial doubt about the Company’s ability to continue as a going concern within one year after the date that the consolidated financial statements are being issued. The Company’s consolidated financial statements do not include any adjustments that might result from the outcome of this uncertainty.
| -57- |
The Company’s ability to continue as a going concern is dependent upon its ability to raise additional equity capital to fund its research and development activities and to ultimately achieve sustainable operating revenues and profitability. The amount and timing of future cash requirements depends on the pace, design and results of the Company’s clinical trial program, which, in turn, depends on the availability of operating capital to fund such activities.
Based on current operating plans, the Company estimates that its existing cash resources at December 31, 2024, and the funds raised subsequent to December 31, 2024, will provide sufficient working capital to fund the current clinical trial program with respect to the development of the Company’s lead anti-cancer clinical compound LB-100 through approximately September 30, 2025. However, existing cash resources will not be sufficient to complete the development of and obtain regulatory approval for the Company’s product candidate, which will require that the Company raise significant additional capital. The Company estimates that it will need to raise additional capital to fund its operations by mid-2025 to be able to proactively manage its current business plan during the remainder of 2025 and during 2026. In addition, the Company’s operating plans may change as a result of many factors that are currently unknown and/or outside of the control of the Company, and additional funds may be needed sooner than planned. The Company is considering various strategies and alternatives to obtain the required additional capital. However, as market conditions present uncertainty as to the Company’s ability to secure additional funds, there can be no assurance that the Company will be able to secure additional financing on acceptable terms, as and when necessary, to continue to conduct operations.
If cash resources are insufficient to satisfy the Company’s ongoing cash requirements, the Company would be required to scale back or discontinue its clinical trial program, as well as its licensing and patent prosecution efforts and its technology and product development efforts, or obtain funds, if available, through strategic alliances, joint ventures or other transaction structures that could require the Company to relinquish rights to and/or control of LB-100, or to curtail or discontinue operations entirely.
Reverse Stock Split
On June 2, 2023, the Company effected a 1-for-10 reverse split of its outstanding shares of common stock. The authorized number of shares of common stock and the par value per share were not affected by the reverse stock split. No fractional shares were issued in connection with the reverse stock split, with all fractional shares being rounded up to the next whole share. All share and per share amounts and information presented herein have been retroactively adjusted to reflect the reverse stock split for all periods presented.
Nasdaq Compliance
The Company’s common stock and the warrants are traded on the Nasdaq Capital Market under the symbols “LIXT” and “LIXTW”, respectively.
On June 2, 2023, the Company effected a 1-for-10 reverse split of its outstanding shares of common stock in order to remain in compliance with the $1.00 minimum closing bid price requirement of Nasdaq. However, there can be no assurances that the Company will be able to remain in compliance with the $1.00 minimum closing bid price requirement of Nasdaq over time. In addition, Nasdaq has other continued listing requirements, one of which is maintaining a minimum net stockholders’ equity of $2,500,000.
On August 23, 2024, the Company received a letter from the Listing Qualifications Department (the “Staff”) of the Nasdaq Stock Market LLC (“Nasdaq”) on August 19, 2024 indicating that the Company was not in compliance with the minimum stockholders’ equity requirement of $2,500,000 for continued listing on the Nasdaq Capital Market under Listing Rule 5550(b) (the “Stockholders’ Equity Requirement”).
On October 3, 2024, the Company submitted a plan to the Staff to regain compliance with the Stockholders’ Equity Requirement, which outlined the Company’s proposed initiatives to regain compliance by raising equity capital through various registered equity offerings.
On October 21, 2024, the Staff provided notice (the “Notice”) to the Company that it had granted an extension through February 18, 2025 to regain compliance with the Stockholders’ Equity Requirement, which required that the Company complete its capital raising initiatives and evidence compliance with the Stockholders’ Equity Requirement through filing a Current Report on Form 8-K with the Securities and Exchange Commission (the “SEC”) providing certain required information.
As of February 18, 2025, the Company had not gained compliance with the Stockholders’ Equity Requirement. Accordingly, on February 19, 2025, the Company received a Staff determination letter from the Staff stating that the Company did not meet the terms of the extension because it did not complete its proposed financing initiatives to regain compliance.
| -58- |
The Company timely filed an appeal and requested a Hearing before a Nasdaq Hearings Panel (the “Panel”), which has been granted. The Hearing request automatically stayed Nasdaq’s delisting of the Company’s common shares and warrants pending the Panel’s decision. Pursuant to the Nasdaq Listing Rules, the Panel has the discretion to grant the Company an additional extension through no later than August 18, 2025. At the upcoming hearing, the Company will present its plan for regaining and sustaining compliance with the Stockholders’ Equity Requirement for continued listing. However, there can be no assurances that the Hearings Panel will grant the Company an extension of time to regain compliance, or that the Company will be able to regain compliance during any extension period. During the appeal process the Company’s common shares and warrants will continue to trade on The Nasdaq Capital Market.
The Company intends to take reasonable measures available to regain compliance under Nasdaq’s listing rules and to remain listed on Nasdaq. However, there can be no assurances that the Company will ultimately regain compliance with the Stockholders’ Equity Rule, or be able to maintain compliance with all other applicable requirements for continued listing on Nasdaq. If the Company does not regain compliance with Nasdaq’s continued listing requirements within the time period permitted by Nasdaq, then the Company’s securities will be delisted from Nasdaq.
Recent Accounting Pronouncements
A description of recently issued accounting pronouncements that may potentially impact the Company’s consolidated financial statements, including their presentation and related disclosures, is provided in Note 2 to consolidated financial statements included elsewhere in this document.
Concentration of Risk
The Company periodically contracts with vendors and consultants to provide services related to the Company’s operations. Charges incurred for these services can be for a specific period (typically one year) or for a specific project or task. Costs and expenses incurred that represented 10% or more of general and administrative costs or research and development costs for the years ended December 31, 2024 and 2023 are described below.
General and administrative costs for the years ended December 31, 2024 and 2023 include charges from legal firms and other vendors for general licensing and patent prosecution costs relating to the Company’s intellectual properties representing 8.6% and 23.3% of total general and administrative costs, respectively. General and administrative costs for the year ended December 31, 2024 also include charges from two vendors and consultants representing 15.0% and 13.1%, respectively, of total general and administrative costs. General and administrative costs for the year ended December 31, 2023 also include charges from a vendor and consultant representing 10.4% of total general and administrative costs. General and administrative costs for the years ended December 31, 2024 and 2023 also included charges for the fair value of stock options granted to directors and corporate officers representing 14.7% and 18.4%, respectively, of total general and administrative costs.
Research and development costs for the year ended December 31, 2024 include charges from three vendors and consultants representing 39.2%, 29.0% and 15.4%, respectively, of total research and development costs. Research and development costs for the year ended December 31, 2023 include charges from three vendors and consultants representing 29.9%, 25.2% and 13.7%, respectively, of total research and development costs.
Critical Accounting Policies and Estimates
The preparation of financial statements in conformity with GAAP requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities at the date of the financial statements and the reported amounts of expenses during the reporting period. Some of those judgments can be subjective and complex, and therefore, actual results could differ materially from those estimates under different assumptions or conditions. Management bases its estimates on historical experience and on various assumptions that are believed to be reasonable in relation to the financial statements taken, as a whole, under the circumstances, the results of which form the basis for making judgments about the carrying values of assets and liabilities that are not readily apparent from other sources. Management regularly evaluates the key factors and assumptions used to develop the estimates utilizing currently available information, changes in facts and circumstances, historical experience, and reasonable assumptions. After such evaluations, if deemed appropriate, those estimates are adjusted accordingly. Actual results could differ from those estimates. Significant estimates include those related to assumptions used in the calculation of accruals for clinical trial costs and other potential liabilities, and valuing equity instruments issued for services.
The following critical accounting policies affect the more significant judgements and estimates used in the preparation of the Company’s consolidated financial statements.
| -59- |
Cash
Cash is held in a cash bank deposit program maintained by Morgan Stanley Wealth Management, a division of Morgan Stanley Smith Barney LLC (“Morgan Stanley”). Morgan Stanley is a FINRA-regulated broker-dealer. The Company’s policy is to maintain its cash balances with financial institutions in the United States with high credit ratings and in accounts insured by the Federal Deposit Insurance Corporation (the “FDIC”) and/or by the Securities Investor Protection Corporation (the “SIPC”). The Company periodically has cash balances in financial institutions in excess of the FDIC and SIPC insurance limits of $250,000 and $500,000, respectively. Morgan Stanley Wealth Management also maintains supplemental insurance coverage for the cash balances of its customers. The Company has not experienced any losses to date resulting from this policy.
Segment Information
The Company’s President and Chief Executive Officer is the Company’s Chief Operating Decision Maker (“CODM”) and evaluates performance and makes operating decisions about allocating resources based on internal financial data presented on a consolidated basis. Because the CODM evaluates financial performance on a consolidated basis, the Company has determined that it operates in a single reportable segment, which consists of the development of a drug class called Protein Phosphatase 2A inhibitors, and is comprised of the consolidated financial results of the Company. The CODM uses consolidated net income (loss) as the sole measure of segment profit or loss.
In November 2023, the Financial Accounting Standards Board (“FASB”) issued Accounting Standards Update (“ASU”) 2023-07, Segment Reporting (Topic 280): Improvements to Reportable Segment Disclosure. ASU 2023-07 amends the FASB Accounting Standards Codification to require additional reportable segment disclosures of a public entity by requiring disclosure of significant segment expenses that are regularly provided to the chief operating decision maker, requiring other new disclosures, and requiring enhanced interim disclosures. ASU 2023-07 requires public entities with a single reportable segment to provide all the disclosures required by ASU 2023-07 and all existing segment disclosures in Topic 280 on an interim and annual basis. The Company adopted ASU 2023-07 effective January 1, 2024 for the 2024 annual period on a retrospective basis. The adoption of ASU 2023-07 resulted in additional required segment-related disclosures in the Company’s financial statements.
Research and Development
Research and development costs consist primarily of fees paid to consultants and contractors, and other expenses relating to the negotiation, design, development, conduct and management of clinical trials with respect to the Company’s clinical compound and product candidate. Research and development costs also include the costs to manufacture compounds used in research and clinical trials, which are charged to operations as incurred. The Company’s inventory of LB-100 for clinical use has been manufactured separately in the United States and in the European Union in accordance with the laws and regulations of such jurisdictions.
Research and development costs are generally charged to operations ratably over the life of the underlying contracts, unless the achievement of milestones, the completion of contracted work, the termination of an agreement, or other information indicates that a different expensing schedule is more appropriate. However, payments for research and development costs that are contractually defined as non-refundable are charged to operations as incurred.
Obligations incurred with respect to mandatory scheduled payments under agreements with milestone provisions are recognized as charges to research and development costs in the Company’s consolidated statement of operations based on the achievement of such milestones, as specified in the respective agreement. Obligations incurred with respect to mandatory scheduled payments under agreements without milestone provisions are accounted for when due, are recognized ratably over the appropriate period, as specified in the respective agreement, and are recorded as liabilities in the Company’s consolidated balance sheet, with a corresponding charge to research and development costs in the Company’s consolidated statement of operations.
| -60- |
Payments made pursuant to contracts are initially recorded as advances on research and development contract services in the Company’s consolidated balance sheet and are then charged to research and development costs in the Company’s consolidated statement of operations as those contract services are performed. Expenses incurred under contracts in excess of amounts advanced are recorded as research and development contract liabilities in the Company’s consolidated balance sheet, with a corresponding charge to research and development costs in the Company’s consolidated statement of operations. The Company reviews the status of its various clinical trial and research and development contracts on a quarterly basis.
Patent and Licensing Legal and Filing Fees and Costs
Due to the significant uncertainty associated with the successful development of commercially viable products based on the Company’s research efforts and related patent applications, all patent and licensing legal and filing fees and costs related to the development and protection of the Company’s intellectual property are charged to operations as incurred. Patent and licensing legal and filing fees and costs are included in general and administrative costs in the Company’s consolidated statement of operations.
In September 2023, the Company appointed a new President and Chief Executive Officer, who, with the assistance of the Company’s management, Board of Directors and patent legal counsel, conducted a comprehensive review and analysis of the Company’s extensive patent portfolio in order to implement a program to balance patent prosecution costs with intellectual property protection benefits. As a result of such review and analysis, the Company identified certain patent filings that it decided not to continue to support in 2024 and thereafter. In addition, the Company changed patent legal counsel in mid-2024. The Company expects that patent and licensing legal and filing fees and costs will continue to be a significant continuing cost in 2025 and thereafter as the Company continues to develop and expand its patent portfolio related to the clinical development of LB-100.
As a result of such review and analysis, patent and licensing legal and filing fees and costs related to the development and protection of the Company’s intellectual property, primarily related to LB-100, decreased to $243,186 for the year ended December 31, 2024, as compared to $978,244 for the year ended December 31, 2023, a decrease of $735,058, or 75.1%.
A descriptive summary of the patent portfolio for the Company’s most important clinical programs involving the development of LB-100, as well as a detailed listing of each domestic and international patent that has been issued, is presented at “ITEM 1. BUSINESS – Intellectual Property”.
Stock-Based Compensation
The Company periodically issues common stock and stock options to officers, directors, employees, contractors and consultants for services rendered. Options vest and expire according to terms established at the issuance date of each grant. Stock grants, which are generally time vested, are measured at the grant date fair value and charged to operations ratably over the vesting period.
The Company accounts for stock-based payments to officers, directors, employees, contractors, and consultants by measuring the cost of services received in exchange for equity awards utilizing the grant date fair value of the awards, with the cost recognized as compensation expense on the straight-line basis in the Company’s financial statements over the vesting period of the awards. Recognition of compensation expense for non-employees is in the same period and manner as if the Company had paid cash for the services.
The fair value of stock options granted as stock-based compensation is determined utilizing the Black-Scholes option-pricing model, and is affected by several variables, the most significant of which are the expected life of the stock option, the exercise price of the stock option as compared to the fair market value of the common stock on the grant date, and the estimated volatility of the common stock. Unless sufficient historical exercise data is available, the expected life of the stock option is calculated as the mid-point between the vesting period and the contractual term (the “simplified method”). The estimated volatility is based on the historical volatility of the Company’s common stock, calculated utilizing a look-back period approximately equal to the contractual life of the stock option being granted. The risk-free interest rate is based on the U.S. Treasury yield curve in effect at the time of grant. The fair market value of the common stock is determined by reference to the quoted market price of the Company’s common stock on the grant date. The expected dividend yield is based on the Company’s expectation of dividend payouts and is assumed to be zero.
| -61- |
The Company recognizes the fair value of stock-based compensation awards in general and administrative costs and in research and development costs, as appropriate, in the Company’s consolidated statements of operations. The Company issues new shares of common stock to satisfy stock option exercises.
Warrants
The Company accounts for warrants as either equity-classified or liability-classified instruments based on an assessment of the warrant’s specific terms and applicable authoritative guidance in Accounting Standards Codification (“ASC”) 480, Distinguishing Liabilities from Equity (“ASC 480”), and ASC 815, Derivatives and Hedging (“ASC 815”). The assessment considers whether the warrants are freestanding financial instruments pursuant to ASC 480, meet the definition of a liability pursuant to ASC 480, and whether the warrants meet all of the requirements for equity classification under ASC 815, including whether the warrants are indexed to the Company’s own common stock and whether the warrant holders could potentially require “net cash settlement” in a circumstance outside of the Company’s control, among other conditions for equity classification. The Company has determined that the warrants issued in the July 20, 2023 equity financing meet the requirements for equity classification. This assessment, which requires the use of professional judgment, is conducted when the warrants are issued and at the end each subsequent quarterly period while the warrants are outstanding. For issued or modified warrants that meet all of the criteria for equity classification, the warrants are required to be recorded as a component of additional paid-in capital at the time of issuance. For issued or modified warrants that do not meet all of the criteria for equity classification, the warrants are required to be liability-classified and recorded at their initial fair value on the date of issuance and remeasured at fair value at each balance sheet date thereafter. Changes in the estimated fair value of the warrants that are liability-classified are recognized as a non-cash gain or loss in the statement of operations at each balance sheet date. At December 31, 2024 and 2023, the Company did not have any liability-classified warrants.
Summary of Business Activities and Plans
Company Overview
The Company is a clinical-stage biopharmaceutical company focused on identifying new targets for cancer drug development and developing and commercializing cancer therapies. The Company’s product pipeline is primarily focused on inhibitors of protein phosphatase 2A, which is used to enhance cytotoxic agents, radiation, immune checkpoint blockers and other cancer therapies. The Company believes that inhibitors of protein phosphatases have significant therapeutic potential for a broad range of cancers. The Company is focusing on the clinical development of a specific protein phosphatase inhibitor, referred to as LB-100, which has been shown to have clinical anti-cancer activity.
The Company believes that the mechanism by which LB-100 affects cancer cell growth is different from cancer agents currently approved for clinical use. LB-100 is currently being tested in clinical trials in Ovarian Clear Cell Carcinoma, Metastatic Micro Satellite Stable (MSS) Colon Cancer, and Advanced Soft Tissue Sarcoma. LB-100 has shown anti-cancer activity in animal models of glioblastoma multiforme, neuroblastoma, and medulloblastoma, all cancers of neural tissue. LB-100 has also been shown to enhance the effectiveness of commonly used anti-cancer drugs in animal models of melanoma, breast cancer and sarcoma. The enhancement of anti-cancer activity of these anti-cancer drugs occurs at doses of LB-100 that do not significantly increase toxicity in animals. It is therefore hoped that, when combined with standard anti-cancer regimens against many tumor types, LB-100 will improve therapeutic benefit.
As a compound moves through the FDA-approval process, it becomes an increasingly valuable property, but at a cost of additional investment at each stage. As the potential effectiveness of LB-100 has been documented at the clinical trial level, the Company has allocated resources to expand the breadth and depth of its patent portfolio. The Company’s approach has been to operate with a minimum of overhead, moving compounds forward as efficiently and inexpensively as possible, and to raise funds to support each of these stages as certain milestones are reached. The Company’s longer-term objective is to secure one or more strategic partnerships or licensing agreements with pharmaceutical companies with major programs in cancer.
| -62- |
Specific Risks Associated with the Company’s Business Activities
Serious Adverse Events
The Company’s lead drug candidate, LB-100, is currently undergoing various clinical trials, and there is a risk that one or more of these trials could be placed on hold by regulatory authorities due to serious adverse events (SAEs) related to the Company’s drug candidate or to another company’s drug used in combination in one of the Company’s clinical trials. It is possible that the SAEs could be attributable to the Company’s drug candidate and could include, but not be limited to, unexpected severe side effects, treatment-related deaths, or long-term health complications. A dose given could result in non-tolerable adverse events defined as dose-limiting toxicity (DLT). When two DLTs occur at the same dose-level, that dose-level is considered too high and unsafe. Further treatment is only allowed at lower dose-levels that have previously been found safe.
If an SAE or a pattern of SAEs is observed during the course of a clinical trial involving the Company’s drug candidate, the U.S. Food and Drug Administration (FDA), European Medicines Agency (EMA), or other regulatory authorities may issue a clinical hold, requiring the Company to pause or discontinue further enrollment and dosing in its clinical trial. It is also possible that the clinical trial could be terminated. Any of these actions could delay or halt the development of the Company’s drug candidate, increase development costs, and negatively impact the Company’s ability to ultimately achieve regulatory approval. Additionally, if an SAE is confirmed to be drug-related, the Company may be required to conduct additional studies, modify the study design, or abandon further development of the drug candidate altogether, which could materially impact the Company’s business, financial condition, and prospects.
The occurrence of an SAE and any resulting clinical hold could also harm the Company’s reputation with patients, physicians, health institutions, and investors, diminish its ability to attract clinical trial participants, and damage its ability to interest investors and obtain financing in the future. There can be no assurance that the Company will not experience such SAEs in the future or that any related clinical hold will be lifted in a timely manner, or at all.
The principal investigator of the colorectal study testing LB-100 in combination with atezolizumab (Roche PD-L1 inhibitor) is currently investigating two SAEs observed in the clinical trial that was launched in August 2024. The Institutional Review Board (the “IRB”) of the Netherlands Cancer Institute (“NKI”) has put the colorectal cancer study on hold. The adverse reactions that developed in the two patients were dyspnea (shortness of breath) due to lung toxicity possibly or probably related to the combination of LB-100 and atezolizumab in one patient and fever and aphasia possibly or probably related to the combination of LB-100 and atezolizumab in the second patient. The patient who developed lung toxicity deceased due to the combination of lung metastases of colorectal cancer and dyspnea. The patient with fever and aphasia fully recovered from the adverse events with supportive medication.
Given the identified adverse events in the two patients in the clinical trial, the IRB requested from the principal investigator of the study at the NKI information as to whether the adverse events could have been caused by the combination of LB-100 and atezolizumab and information about the mode of action of the combination of LB-100 and atezolizumab. The principal investigator is preparing a response to the IRB detailing the safety experience with LB-100 given alone and in combination with other cancer drugs, especially doxorubicin and dostarlimab. Doxorubicin is a well-known chemotherapy, and dostarlimab is a well-known immunotherapy of which the mode of action is closely related to that of atezolizumab.
The reported adverse events in the colorectal cancer study have not been seen in any other patients thus far treated with LB-100 alone or in combination with other cancer drugs. Through February 2025, a total of 78 patient have received or are receiving experimental treatment with LB-100. It is expected that it will take at least two months to prepare a detailed response to the IRB, during which time the Company intends to update the safety overview of LB-100.
External Risks Associated with the Company’s Business Activities
Covid-19 Virus. The global outbreak of the novel coronavirus (Covid-19) in early 2020 led to disruptions in general economic activities throughout the world as businesses and governments implemented broad actions to mitigate this public health crisis. Although Covid-19 outbreak has subsided, the extent to which the coronavirus pandemic may reappear and impact the Company’s clinical trial programs and capital raising efforts in the future is uncertain and cannot be predicted.
| -63- |
Inflation and Interest Rate Risk. The Company does not believe that inflation or increasing interest rates have had a material effect on its operations to date, other than their impact on the general economy. However, there is a risk that the Company’s operating costs could become subject to inflationary and interest rate pressures in the future, which would have the effect of increasing the Company’s operating costs (including, specifically, clinical trial costs), and which would put additional stress on the Company’s working capital resources.
Supply Chain Issues. The Company does not currently expect that supply chain issues will have a significant impact on its business activities, including its ongoing clinical trials.
Potential Recession. There are some indications that the United States economy may be at risk of entering a recessionary period. Although unclear at this time, an economic recession would likely impact the general business environment and the capital markets, which could, in turn, affect the Company.
Geopolitical Risk. The geopolitical landscape poses inherent risks that could significantly impact the operations and financial performance of the Company. In the event of a military conflict, supply chain disruptions, geopolitical uncertainties, and economic repercussions may adversely affect the Company’s ability to conduct research, develop, test and manufacture products, and distribute them globally. This could lead to delays in product development, interruptions in the supply of critical materials, and delays in clinical trials, thereby impeding the Company’s clinical development and commercialization plans. Furthermore, the impact of a conflict on global financial markets may result in increased volatility and uncertainty in the capital markets, thereby affecting the valuation of the Company’s publicly-traded shares. Investor confidence, market sentiment, and access to capital could all be negatively influenced. Such geopolitical risks are outside the control of the Company, and the actual effects on the Company’s business, financial condition and results of operations may differ from current estimates.
Cybersecurity Risks. The Company has established policies and processes for assessing, identifying and managing material risk from cybersecurity threats, and has integrated these processes into its overall risk management systems and processes. The Company routinely assesses material risks from cybersecurity threats, including any potential unauthorized occurrence on or conducted through its information and email systems that may result in adverse effects on the confidentiality, integrity, or availability of the Company’s information and email systems or any information residing therein. The Company conducts periodic risk assessments to identify cybersecurity threats, as well as assessments in the event of a material change in the Company’s business practices that may affect information systems that are vulnerable to such cybersecurity threats. These risk assessments include identification of reasonably foreseeable internal and external risks, the likelihood and potential damage that could result from such risks, and the sufficiency of existing policies, procedures, systems and safeguards in place to manage such risks. The Company has not encountered any cybersecurity challenges to date that have materially impaired its operations or financial condition.
The Company is continuing to monitor these matters and will adjust its current business and financing plans as more information becomes available.
Results of Operations
At December 31, 2024, the Company had not yet commenced any revenue-generating operations, does not have any positive cash flows from operations, and is dependent on its ability to raise equity capital to fund its operating requirements.
| -64- |
The Company’s consolidated statements of operations as discussed herein are presented below.
| Years Ended December 31, | ||||||||
| 2024 | 2023 | |||||||
| Revenues | $ | — | $ | — | ||||
| Costs and expenses: | ||||||||
| Research and development costs | 726,232 | 898,100 | ||||||
| General and administrative costs | 2,846,557 | 4,192,136 | ||||||
| Total costs and expenses | 3,572,789 | 5,090,236 | ||||||
| Loss from operations | (3,572,789 | ) | (5,090,236 | ) | ||||
| Interest income | 7,048 | 17,486 | ||||||
| Interest expense | (16,821 | ) | (16,233 | ) | ||||
| Foreign currency gain (loss) | (3,403 | ) | 1,954 | |||||
| Net loss | $ | (3,585,965 | ) | $ | (5,087,029 | ) | ||
| Net loss per common share – basic and diluted | $ | (1.59 | ) | $ | (2.66 | ) | ||
| Weighted average common shares outstanding – basic and diluted | 2,249,290 | 1,915,838 | ||||||
Years Ended December 31, 2024 and 2023
Revenues. The Company did not have any revenues for the years ended December 31, 2024 and 2023.
Research and Development Costs. For the year ended December 31, 2024, research and development costs were $726,232, which consisted of clinical and related oversight costs of $377,958, regulatory service costs of $18,836, and preclinical research focused on development of additional novel anti-cancer compounds to add to the Company’s clinical pipeline of $329,438.
Included in clinical and related oversight costs for the year ended December 31, 2024 is $207,004 for the cost of patients enrolled in the City of Hope clinical trial prior to its termination on July 8, 2024.
For the year ended December 31, 2023, research and development costs were $898,100, which consisted of clinical and related oversight costs of $416,269, regulatory service costs of $18,738, and preclinical research focused on development of additional novel anti-cancer compounds to add to the Company’s clinical pipeline of $463,093.
Effective June 10, 2024, the Company entered into a Clinical Trial Agreement with the Netherlands Cancer Institute (“NKI”) to conduct a Phase 1b/2 clinical trial of the Company’s protein phosphatase inhibitor, LB-100, combined with atezolizumab, a PD-L1 inhibitor, the proprietary molecule of F. Hoffman-La Roche Ltd. (“Roche”), for patients with metastatic colon cancer. NKI employs Dr. René Bernards, a director of the Company since June 15, 2022. The Company has no financial contractual commitment associated with this clinical trial.
Included in preclinical research costs for the years ended December 31, 2024 and 2023 were $210,362 and $226,150, respectively, of costs paid to the Netherlands Cancer Institute, On October 8, 2021, the Company entered into a Development Collaboration Agreement with the Netherlands Cancer Institute, Amsterdam, one of the world’s leading comprehensive cancer centers, and Oncode Institute, Utrecht, a major independent cancer research center, to identify the most promising drugs to be combined with LB-100, and potential LB-100 analogues, to be used to treat a range of cancers, as well as to identify the specific molecular mechanisms underlying the identified combinations.
On October 3, 2023, the Company entered into Amendment No. 2 to the Development Collaboration Agreement with the Netherlands Cancer Institute, which provided for additional research activities, extended the termination date of the Development Collaboration Agreement by two years to October 8, 2026, and added 500,000 Euros to the operating budget being funded by the Company.
On October 4, 2024, the Company entered into Amendment No. 3 to the Development Collaboration Agreement with NKI, which suspended Amendment No. 2 and provided for a new study term of one year commencing upon the dosing of the first patient in the clinical trial at a project cost of 100,000 Euros (see “Principal Commitments – Other Significant Agreements and Contracts – Netherlands Cancer Institute” below).
Research and development costs decreased by $171,868, or 19.1%, in 2024 as compared to 2023, primarily as a result of a decrease in preclinical research focused on development of additional novel anti-cancer compounds to add to the Company’s clinical pipeline of $133,655.
| -65- |
General and Administrative Costs. For the year December 31, 2024, general and administrative costs were $2,846,557, which consisted of the fair value of vested stock options issued to directors and officers of $418,422 (including quarterly director and board committee fees of $55,000), patent and licensing legal and filing fees and costs of $243,186, other consulting and professional fees of $735,021, insurance expense of $434,444, officer salaries and related costs of $691,244, cash-based director and board committee fees of $38,819, licensing and royalties of $75,643, shareholder reporting costs of $41,488, listing fees of $49,500, filing fees of $28,012, investor relations of $59,588, rent of $16,435, conference fees of $14,475 and other operating costs of $45,830, offset by a state franchise tax credits of $45,550.
For the year ended December 31, 2023, general and administrative costs were $4,192,136, which consisted of the fair value of vested stock options issued to directors and officers of $773,203, patent and licensing legal and filing fees and costs of $978,244, other consulting and professional fees of $655,854, insurance expense of $442,976, officer salaries and related costs of $841,709, cash-based director and board committee fees of $163,479, shareholder reporting costs of $93,860, listing fees of $62,000, filing fees of $17,125, taxes and licenses of $73,877, investor relations of $59,238, rent of $15,571 and other operating costs of $24,109, offset by a credit to licensing fees of $9,109 relating to the termination of the Moffitt agreement.
General and administrative costs decreased by $1,345,579, or 32.1%, in 2024 as compared to 2023, primarily as a result of a decrease in the fair value of vested stock options issued to directors and officers of $354,781, a decrease in patent and licensing legal and filing fees and costs of $735,058, a decrease in officer salaries and related costs of $150,465, a decrease in shareholder reporting costs of $52,372, a decrease in taxes and licenses of $119,427, and a decrease in cash-based director and board committee fees of $124,660, offset by increases in licensing and royalties of $84,752, and in other consulting and professional fees of $79,167.
Interest Income. For the year ended December 31, 2024, the Company had interest income of $7,048, as compared to interest income of $17,486 for the year ended December 31, 2023, related to the investment of the Company’s cash resources.
Interest Expense. For the year ended December 31, 2024, the Company had interest expense of $16,821, as compared to interest expense of $16,233 for the year ended December 31, 2023, related to the financing of the premium for the Company’s directors and officers liability insurance policy.
Foreign Currency Gain (Loss). For the year ended December 31, 2024, the Company had a foreign currency loss of $3,403, as compared to a foreign currency gain of $1,954 for the year ended December 31, 2023, from foreign currency transactions.
Net Loss. For the year ended December 31, 2024, the Company incurred a net loss of $3,585,965, as compared to a net loss of $5,087,029 for the year ended December 31, 2023.
Liquidity and Capital Resources – December 31, 2024
The Company’s consolidated statements of cash flows as discussed herein are as follows:
| Years Ended December 31, | ||||||||
| 2024 | 2023 | |||||||
| Net cash used in operating activities | $ | (3,164,536 | ) | $ | (4,293,265 | ) | ||
| Net cash provided by (used in) investing activities | — | — | ||||||
| Net cash provided by financing activities | — | 3,143,361 | ||||||
| Net decrease in cash | $ | (3,164,536 | ) | $ | (1,146,904 | ) | ||
At December 31, 2024, the Company had working capital of $827,219, as compared to working capital of $3,994,762 at December 31, 2023, reflecting a decrease in working capital of $3,167,543 for the year ended December 31, 2024. The decrease in working capital during the year ended December 31, 2024 was primarily the result of the funding of the Company’s ongoing research and development activities and other ongoing operating expenses, including maintaining and developing the Company’s patent portfolio. At December 31, 2024, the Company had cash of $1,038,952 available to fund its operations. Subsequently, the Company completed a securities offering that generated gross proceeds of $1,050,003 during February 2025 before deducting the placement agent’s fees and related offering expenses.
| -66- |
Going Concern
The Company’s consolidated financial statements have been presented on the basis that it will continue as a going concern, which contemplates the realization of assets and satisfaction of liabilities in the normal course of business. The consolidated financial statements also do not reflect any adjustments relating to the recoverability of assets and liabilities that might be necessary if the Company is unable to continue as a going concern. The Company has no recurring source of revenues and has experienced negative operating cash flows since inception. The Company has financed its working capital requirements through the recurring sale of its equity securities.
Based on the foregoing, management has concluded that there is substantial doubt about the Company’s ability to continue as a going concern within one year after the date that the consolidated financial statements are being issued. In addition, the Company’s independent registered public accounting firm has included an explanatory paragraph in their report with respect to this uncertainty that accompanies the Company’s audited consolidated financial statements as of and for the year ended December 31, 2024. The Company’s independent registered public accounting firm, in their report on the Company’s December 31, 2024 audited consolidated financial statements, has expressed substantial doubt about the Company’s ability to continue as a going concern. The Company’s consolidated financial statements do not include any adjustments that might result from the outcome of this uncertainty.
The Company’s ability to continue as a going concern is dependent upon its ability to raise additional equity capital to fund its research and development activities and to ultimately achieve sustainable operating revenues and profitability. The amount and timing of future cash requirements depends on the pace, design, and results of the Company’s clinical trial program, which, in turn, depends on the availability of operating capital to fund such activities.
Based on current operating plans, the Company estimates that its existing cash resources at December 31, 2024, and the funds raised subsequent to December 31, 2024, will provide sufficient working capital to fund the current clinical trial program with respect to the development of the Company’s lead anti-cancer clinical compound LB-100 through approximately September 30, 2025. However, existing cash resources will not be sufficient to complete the development of and obtain regulatory approval for the Company’s product candidate, which will require that the Company raise significant additional capital. The Company estimates that it will need to raise additional capital to fund its operations by mid-2025 to be able to proactively manage its current business plan during the remainder of 2025 and during 2026. In addition, the Company’s operating plans may change as a result of many factors that are currently unknown and/or outside of the control of the Company, and additional funds may be needed sooner than planned. The Company is considering various strategies and alternatives to obtain the required additional capital. However, as market conditions present uncertainty as to the Company’s ability to secure additional funds, there can be no assurance that the Company will be able to secure additional financing on acceptable terms, as and when necessary, to continue to conduct operations.
If cash resources are insufficient to satisfy the Company’s ongoing cash requirements, the Company would be required to scale back or discontinue its clinical trial program, as well as its licensing and patent prosecution efforts and its technology and product development efforts, or obtain funds, if available, through strategic alliances, joint ventures or other transaction structures that could require the Company to relinquish rights to and/or control of LB-100, or to curtail or discontinue operations entirely.
At March 14, 2025, the Company’s remaining financial contractual commitments pursuant to clinical trial agreements and clinical trial monitoring agreements not yet incurred aggregated $526,000, which are currently scheduled to be incurred through approximately December 31, 2027.
At December 31, 2024, the Company did not have any transactions, obligations or relationships that could be considered off-balance sheet arrangements.
Operating Activities. For the year ended December 31, 2024, operating activities utilized cash of $3,164,536, as compared to utilizing cash of $4,293,265 for the year ended December 31, 2023, to fund the Company’s ongoing research and development activities and to fund its other ongoing operating expenses, including maintaining and developing its patent portfolio.
Investing Activities. For the years ended December 31, 2024 and 2023, the Company had no investing activities.
Financing Activities. For the year ended December 31, 2024, the Company had no financing activities. For the year ended December 31, 2023, financing activities consisted primarily of the gross proceeds from the sale of securities in the Company’s registered direct offering of $3,499,964, reduced by offering costs of $362,925, and $6,281 from the exercise of common stock options.
| -67- |
Principal Commitments
Clinical Trial Agreements
At March 14, 2025, the Company’s remaining financial contractual commitments pursuant to clinical trial agreements and clinical trial monitoring agreements not yet incurred, as described below, aggregated $526,000, including clinical trial agreements of $264,000 and clinical trial monitoring agreements of $262,000, which, based on current estimates, are currently scheduled to be incurred through approximately December 31, 2027. The Company’s ability to conduct and fund these contractual commitments is subject to the timely availability of sufficient capital to fund such expenditures, as well as any changes in the allocation or reallocation of such funds to the Company’s current or future clinical trial programs. The Company expects that the full amount of these expenditures will be incurred only if such clinical trial programs are conducted as originally designed and their respective enrollments and duration are not modified or reduced. Clinical trial programs, such as the types that the Company is engaged in, can be highly variable and can frequently involve a series of changes and modifications over time as clinical data is obtained and analyzed, and is frequently modified, suspended or terminated, in part based on receipt or lack of receipt of an indication of clinical benefit or activity, before the clinical trial endpoint is reached. Accordingly, such contractual commitments as discussed herein should be considered as estimates only based on current clinical assumptions and conditions and are typically subject to significant modifications and revisions over time.
Additional information with respect to the conduct of the Company’s clinical trial programs is provide at “ITEM 1A. RISK FACTORS - Risks Related to the Development and Regulatory Approval of Our Product Candidates”.
The following is a summary of the Company’s ongoing contractual clinical trials described below as of March 14, 2025:
| Description of Clinical Trial | Institution | Start Date | Projected End Date |
Number of Patients in Trial |
Study Objective | Clinical Update |
Expected Date of Preliminary Efficacy Signal |
NCT No. |
Remaining Financial Contractual Commitment |
|||||||||||
| LB-100 combined with atezolizumab in microsatellite stable metastatic colorectal cancer (Phase 1b) | Netherlands Cancer Institute (NKI) | August 2024 | December 2026 | 37 | Determine RP2D with atezolizumab | First patient entered August 2024, in total two patients entered | June 2026 | NCT06012734 | (1 | ) | ||||||||||
| LB-100 combined with doxorubicin in advanced soft tissue sarcoma (Phase 1b) | GEIS | June 2023 | Recruitment completed September 2024 | 9 to 18 | Determine MTD and RP2D | Fourteen patients entered | December 2025 | NCT05809830 | $ | 264,000 | ||||||||||
| Doxorubicin with or without LB-100 in advanced soft tissue sarcoma (Randomized Phase 2) | GEIS | TBD | TBD | 150 | Determine efficacy: PFS | Clinical trial not yet begun (subject to completion of Phase 1b GEIS clinical trial) | TBD | NCT05809830 | $ | (1 | ) | |||||||||
| LB-100 combined with dostarlimab in ovarian clear cell carcinoma (Phase 1b/2) | MD Anderson | January 2024 | December 2027 | 21 | Determine the OS of patients with recurrent ovarian clear cell carcinoma | Nine patients entered | December 2026 | NCT06065462 | (1 | ) | ||||||||||
| Total | $ | 264,000 | ||||||||||||||||||
| (1) | The Company has no financial contractual commitment associated with this clinical trial at March 14, 2025. |
| -68- |
Netherlands Cancer Institute. Effective June 10, 2024, the Company entered into a Clinical Trial Agreement with the Netherlands Cancer Institute (“NKI”) to conduct a Phase 1b clinical trial of the Company’s protein phosphatase inhibitor, LB-100, combined with atezolizumab, a PD-L1 inhibitor, the proprietary molecule of F. Hoffman-La Roche Ltd. (“Roche”), for patients with microsatellite stable metastatic colorectal cancer. Under the agreement, the Company will provide its lead compound, LB-100, and under a separate agreement between NKI and Roche, Roche will provide atezolizumab and financial support for the clinical trial. The Company has no obligation to and will not provide any reimbursement of clinical trial costs. Pursuant to the agreement and the protocol set forth in the agreement, the clinical trial will be conducted by NKI at NKI’s site in Amsterdam by principal investigator Neeltje Steeghs, MD, PhD, and NKI will be responsible for the recruitment of patients. The agreement provides for the protection of the respective intellectual property rights of each of the Company, NKI and Roche.
This Phase 1b clinical trial will evaluate safety, optimal dose and preliminary efficacy of LB-100 combined with atezolizumab for the treatment of patients with metastatic microsatellite stable colorectal cancer. Immunotherapy using monoclonal antibodies like atezolizumab can enhance the body’s immune response against cancer and hinder tumor growth and spread. LB-100 has been found to improve the effectiveness of anticancer drugs in killing cancer cells by inhibiting a protein called PP2A on cell surfaces. Blocking PP2A increases stress signals in tumor cells expressing the PP2A protein. Accordingly, combining atezolizumab with LB-100 may enhance treatment efficacy for metastatic colorectal cancer, as cancer cells with heightened stress signals are more vulnerable to immunotherapy.
This study comprises a dose escalation phase and a dose expansion phase. The objective of the dose escalation phase is to determine the recommended Phase 2 dose (RP2D) of LB-100 when combined with the standard dosage of atezolizumab. The dose expansion phase will further investigate the preliminary efficacy, safety, tolerability, and pharmacokinetics/dynamics of the LB-100 and atezolizumab combination. The clinical trial opened in August 2024 with the enrollment of the first patient. A total of two patients have been enrolled to date. Patient accrual is expected to take up to 24 months, with a maximum of 37 patients with advanced colorectal cancer to be enrolled in this study.
The principal investigator of the colorectal study testing LB-100 in combination with atezolizumab is currently investigating two Serious Adverse Events (“SAEs”) observed in the clinical trial (see “Specific Risks Associated with the Company’s Business Activities – Serious Adverse Events” above for additional information).
The Company has no financial contractual commitment associated with this clinical trial.
City of Hope. Effective January 18, 2021, the Company executed a Clinical Research Support Agreement (the “Agreement”) with the City of Hope National Medical Center, an NCI-designated comprehensive cancer center, and City of Hope Medical Foundation (collectively, “City of Hope”), to carry out a Phase 1b clinical trial of LB-100, the Company’s first-in-class protein phosphatase inhibitor, combined with an FDA-approved standard regimen for treatment of untreated extensive-stage disease small cell lung cancer (“ED-SCLC”). LB-100 was given in combination with carboplatin, etoposide and atezolizumab, an FDA-approved standard of care regimen, to previously untreated ED-SCLC patients. The LB-100 dose was to be escalated with the standard fixed doses of the 3-drug regimen to reach a recommended Phase 2 dose (“RP2D”). Patient entry was to be expanded so that a total of 12 patients would be evaluable at the RP2D to confirm the safety of the LB-100 combination and to look for potential therapeutic activity as assessed by objective response rate, duration of overall response, progression-free survival, and overall survival.
The clinical trial was initiated on March 9, 2021, with patient accrual expected to take approximately two years to complete. Because patient accrual was slower than expected, effective March 6, 2023, the Company and City of Hope added the Sarah Cannon Research Institute (“SCRI”), Nashville, Tennessee, to the ongoing Phase 1b clinical trial. The Company and City of Hope continued efforts to increase patient accrual by adding additional sites and by modifying the protocol to increase the number of patients eligible for the clinical trial. The impact of these efforts to increase patient accrual and to decrease time to completion was evaluated in subsequent quarters.
After evaluating patient accrual through June 30, 2024, the Company and City of Hope agreed to close the clinical trial. Pursuant to the terms of the Agreement, the Company provided notice to City of Hope of the Company’s intent to terminate the Agreement effective as of July 8, 2024. Upon closure, the Company incurred a prorated charge of $207,004 for the cost of patients enrolled to date, which is included in accounts payable and accrued expenses at December 31, 2024.
| -69- |
During the year ended December 31, 2024 and 2023, the Company incurred costs of $285,019 and $69,001, respectively, pursuant to this Agreement. As of December 31, 2024, total costs of $732,532 had been incurred pursuant to this Agreement.
GEIS. Effective July 31, 2019, the Company entered into a Collaboration Agreement for an Investigator-Initiated Clinical Trial with the Spanish Sarcoma Group (Grupo Español de Investigación en Sarcomas or “GEIS”), Madrid, Spain, to carry out a study entitled “Randomized phase I/II trial of LB-100 plus doxorubicin vs. doxorubicin alone in first line of advanced soft tissue sarcoma”. The purpose of this clinical trial is to obtain information with respect to the efficacy and safety of LB-100 combined with doxorubicin in soft tissue sarcomas. Doxorubicin is the global standard for initial treatment of advanced soft tissue sarcomas (“ASTS”). Doxorubicin alone has been the mainstay of first line treatment of ASTS for over 40 years, with little improvement in survival from adding cytotoxic compounds to or substituting other cytotoxic compounds for doxorubicin. In animal models, LB-100 consistently enhances the anti-tumor activity of doxorubicin without apparent increases in toxicity.
GEIS has a network of referral centers in Spain and across Europe that have an impressive track record of efficiently conducting innovative studies in ASTS. The Company agreed to provide GEIS with a supply of LB-100 to be utilized in the conduct of this clinical trial, as well as to provide funding for the clinical trial. The goal is to enter approximately 150 to 170 patients in this clinical trial over a period of two to four years. The Phase 1 portion of the study began in the quarter ended June 30, 2023 to determine the recommended Phase 2 dose of the combination of doxorubicin and LB-100. As advanced sarcoma is a very aggressive disease, the design of the Phase 2 portion of the study assumes a median progression-free survival (“PFS”), no evidence of disease progression or death from any cause, of 4.5 months in the doxorubicin arm and an alternative median PFS of 7.5 months in the doxorubicin plus LB-100 arm to demonstrate a statistically significant decrease in relative risk of progression or death by adding LB-100. There is a planned interim analysis of the primary endpoint when approximately 50% of the 102 events required for final analysis is reached.
The Company had previously expected that this clinical trial would commence during the quarter ended June 30, 2020. However, during July 2020, the Spanish regulatory authority advised the Company that although it had approved the scientific and ethical basis of the protocol, it required that the Company manufacture new inventory of LB-100 under current Spanish pharmaceutical manufacturing standards. These standards were adopted subsequent to the production of the Company’s existing LB-100 inventory.
In order to manufacture a new inventory supply of LB-100 for the GEIS clinical trial, the Company engaged a number of vendors to carry out the multiple tasks needed to make and gain approval of a new clinical product for investigational study in Spain. These tasks included the synthesis under good manufacturing practice (GMP) of the active pharmaceutical ingredient (API), with documentation of each of the steps involved by an independent auditor. The API was then transferred to a vendor that prepares the clinical drug product, also under GMP conditions documented by an independent auditor. The clinical drug product was then sent to a vendor to test for purity and sterility, provide appropriate labels, store the drug, and distribute the drug to the clinical centers for use in the clinical trials. A formal application documenting all steps taken to prepare the clinical drug product for clinical use was submitted to the appropriate regulatory authorities for review and approval before being used in a clinical trial.
As of December 31, 2024, this program to provide new inventory of the clinical drug product for the Spanish Sarcoma Group study, and potentially for subsequent multiple trials within the European Union, had cost approximately $1,144,000.
On October 13, 2022, the Company announced that the Spanish Agency for Medicines and Health Products (Agencia Española de Medicamentos y Productos Sanitarios or “AEMPS”) had authorized a Phase 1b/randomized Phase 2 study of LB-100, the Company’s lead clinical compound, plus doxorubicin, versus doxorubicin alone, the global standard for initial treatment of ASTS. Consequently, this clinical trial commenced during the quarter ended June 30, 2023 and is expected to be completed and a report prepared by December 31, 2026. In April 2023, GEIS completed its first site initiation visit in preparation for the clinical trial at Fundación Jiménez Díaz University Hospital (Madrid). Up to 170 patents will be entered into the clinical trial. The recruitment for the Phase 1b portion of the protocol was extended with two patients and was completed during the quarter ended September 30, 2024. The Company expects to have data on toxicity and preliminary efficacy from this portion of the clinical trial during the quarter ending December 31, 2025.
| -70- |
Given the focus on the combination of LB-100 with immunotherapy in ovarian clear cell carcinoma and colorectal cancer and the availability of capital resources, the Company entered into Amendment No. 1 to the Collaboration Agreement effective March 11, 2025 that relieved the Company of the financial obligation to support the randomized Phase 2 portion of the clinical trial contemplated in the Collaboration Agreement of approximately $3,095,000. As a result, it is uncertain as to whether the Phase 2 portion of this clinical trial will proceed.
The Company’s agreement with GEIS provided for various payments based on achieving specific milestones over the term of the agreement. During the years ended December 31, 2024 and 2023, the Company incurred costs of $0 and $268,829, respectively, pursuant to this agreement. Through December 31, 2024, the Company has incurred charges of $684,652 for work done under this agreement through the fourth milestone.
The Company’s aggregate commitment pursuant to this agreement, less amounts previously paid to date, totaled approximately $264,000 for the Phase 1b portion of this clinical trial as of March 14, 2025, which is scheduled to be incurred through December 31, 2025. As the work is being conducted in Europe and is paid for in Euros, final costs are subject to foreign currency fluctuations between the United States Dollar and the Euro. Such fluctuations are recorded in the consolidated statements of operations as foreign currency gain or loss, as appropriate, and have not been significant.
MD Anderson Cancer Center Clinical Trial. On September 20, 2023, the Company announced an investigator-initiated Phase 1b/2 collaborative clinical trial to assess whether adding LB-100 to a human programmed death receptor-1 (“PD-1”) blocking antibody of GSK plc (“GSK”), dostarlimab-gxly, may enhance the effectiveness of immunotherapy in the treatment of ovarian clear cell carcinoma (“OCCC”). The study objective is to determine the overall survival (“OS”) of patients with OCCC. The clinical trial is being sponsored by The University of Texas MD Anderson Cancer Center (“MD Anderson”) and is being conducted at The University of Texas - MD Anderson Cancer Center. The Company is providing LB-100 and GSK is providing dostarlimab-gxly and financial support for the clinical trial. On January 29, 2024, the Company announced the entry of the first patient into this clinical trial. The Company currently expects that this clinical trial will be completed by December 31, 2027.
On February 25, 2025, the Company announced that it has added the Robert H. Lurie Comprehensive Cancer Center (Lurie Cancer Center) of Northwestern University as a second site in a clinical trial combining the Company’s proprietary compound LB-100 with GSK’s dostarlimab to treat ovarian clear cell cancer. Patient recruitment is underway, and the first patient has been dosed.
Moffitt. Effective August 20, 2018, the Company entered into a Clinical Trial Research Agreement with the Moffitt Cancer Center and Research Institute Hospital Inc., Tampa, Florida (“Moffitt”), effective for a term of five years. Pursuant to the Clinical Trial Research Agreement, Moffitt agreed to conduct and manage a Phase 1b/2 clinical trial to evaluate the toxicity and therapeutic benefit of the Company’s lead anti-cancer clinical compound LB-100 to be administered intravenously in patients with low or intermediate-1 risk myelodysplastic syndrome (“MDS”).
In November 2018, the Company received approval from the U.S. Food and Drug Administration for its Investigational New Drug (“IND”) Application to conduct a Phase 1b/2 clinical trial to evaluate the toxicity and therapeutic benefit of LB-100 in patients with low and intermediate-1 risk MDS who had failed or were intolerant of standard treatment. This Phase 1b/2 clinical trial utilized LB-100 as a single agent in the treatment of patients with low and intermediate-1 risk MDS.
The clinical trial began at a single site in April 2019 and the first patient was entered into the clinical trial in July 2019. During the year ended December 31, 2023, the clinical trial was closed. Although the maximum tolerated dose (“MTD”) was not achieved, there was no dose-limiting toxicity noted.
During the years ended December 31, 2024 and 2023, the Company incurred costs of $0 and $16,165, respectively, pursuant to this agreement. As of December 31, 2024, total costs of $147,239 had been incurred pursuant to this agreement.
During September 2023, the Company decided not to pursue further studies in MDS, as other, more promising, opportunities had become available (see “Patent and License Agreements - Moffitt” below).
| -71- |
National Cancer Institute Pharmacologic Clinical Trial. In May 2019, the National Cancer Institute (“NCI”) initiated a glioblastoma (“GBM”) pharmacologic clinical trial. This study was being conducted and funded by the NCI under a Cooperative Research and Development Agreement, with the Company responsible for providing the LB-100 clinical compound.
Primary malignant brain tumors (gliomas) are very challenging to treat. Radiation combined with the chemotherapeutic drug temozolomide has been the mainstay of therapy of the most aggressive gliomas (glioblastoma multiforme or GBM) for decades, with little further benefit gained by the addition of one or more anti-cancer drugs, but without major advances in overall survival for the majority of patients. In animal models of GBM, the Company’s novel protein phosphatase inhibitor, LB-100, has been found to enhance the effectiveness of radiation, temozolomide chemotherapy treatments and immunotherapy, raising the possibility that LB-100 may improve outcomes of standard GBM treatment in the clinic. Although LB-100 has proven safe in patients at doses associated with apparent anti-tumor activity against several human cancers arising outside the brain, the ability of LB-100 to penetrate tumor tissue arising in the brain was not known. Many drugs potentially useful for GBM treatment do not enter the brain in amounts necessary for anti-cancer action.
The NCI study was designed to determine the extent to which LB-100 enters recurrent malignant gliomas. Patients having surgery to remove one or more tumors received one dose of LB-100 prior to surgery and had blood and tumor tissue analyzed to determine the amount of LB-100 present and to determine whether the cells in the tumors showed the biochemical changes expected to be present if LB-100 reached its molecular target. As a result of the innovative design of the NCI study, it was believed that data from a few patients would be sufficient to provide a sound rationale for conducting a larger clinical trial to determine the effectiveness of adding LB-100 to the standard treatment regimen for GBMs. Blood and brain tumor tissue were analyzed from seven patients after intravenous infusion of a single dose of LB-100. Results of the investigation demonstrated that there was virtually no entry of LB-100 into the brain tumor tissue. Accordingly, alternative methods of drug delivery will be required to determine if LB-100 has meaningful clinical anti-cancer activity against glioblastoma multiforme and other aggressive brain tumors.
Clinical Trial Monitoring Agreements
MD Anderson Cancer Center Clinical Trial. On May 15, 2024, the Company signed a letter of intent with Theradex to monitor the MD Andersen investigator-initiated Phase 1b/2 collaborative clinical trial to assess whether adding LB-100 to a human programmed death receptor-1 (“PD-1”) blocking antibody of GSK plc (“GSK”), dostarlimab-gxly, may enhance the effectiveness of immunotherapy in the treatment of ovarian clear cell carcinoma (“OCCC”). On August 19, 2024, the Company signed a work order agreement with Theradex to monitor the MD Anderson clinical trial. The study oversight is expected to be completed by January 31, 2027.
Costs under this letter of intent and related work order agreement are estimated to be approximately $95,000. During the year ended December 31, 2024, the Company incurred costs of $26,763 pursuant to this letter of intent and subsequent work order. As of December 31, 2024, total costs of $26,763 have been incurred pursuant to this letter of intent and subsequent work order.
The Company’s aggregate commitment pursuant to this letter of intent, less amounts previously paid to date, totaled approximately $70,000 as of December 31, 2024, which is expected to be incurred through December 31, 2027.
City of Hope. On February 5, 2021, the Company signed a new work order agreement with Theradex to monitor the City of Hope investigator-initiated clinical trial in small cell lung cancer in accordance with FDA requirements for oversight by the sponsoring party. Costs under this work order agreement were estimated to be approximately $335,000. During the years December 31, 2024 and 2023, the Company incurred costs of $10,642 and $20,240, respectively, pursuant to this work order. As of December 31, 2024, total costs of $89,323 had been incurred pursuant to this work order agreement.
As a result of the closure of the Agreement with City of Hope effective July 8, 2024 (see “Clinical Trial Agreements – City of Hope” above), the work order agreement with Theradex to monitor this clinical trial was concurrently terminated, although nominal oversight trailing costs subsequent to July 8, 2024 are expected to be incurred relating to the closure of this study.
| -72- |
GEIS. On June 22, 2023, the Company finalized a work order agreement with Theradex, to monitor the GEIS investigator-initiated clinical Phase I/II randomized trial of LB-100 plus doxorubicin vs. doxorubicin alone in first line of advanced soft tissue sarcoma. The study oversight is expected to be completed by December 31, 2026.
Costs under this work order agreement are estimated to be approximately $153,000, with such payments expected to be allocated approximately 72% to Theradex for services and approximately 28% for payments for pass-through software costs. During the years ended December 31, 2024 and 2023, the Company incurred costs of $34,593 and $14,862, respectively, pursuant to this work order. As of December 31, 2024, total costs of $49,455 have been incurred pursuant to this work order agreement.
The Company’s aggregate commitment pursuant to this clinical trial monitoring agreement, less amounts previously paid to date, totaled approximately $104,000 as of December 31, 2024, which is expected to be incurred through December 31, 2026.
Netherlands Cancer Institute. On August 27, 2024, the Company finalized a work order agreement with Theradex, to monitor the NKI Phase 1b clinical trial of LB-100 combined with atezolizumab, a PD-L1 inhibitor, for patients with microsatellite stable metastatic colorectal cancer. The study oversight is expected to be completed by May 31, 2027.
Costs under this work order agreement are estimated to be approximately $106,380, with such payments expected to be allocated approximately 47% to Theradex for services and approximately 53% for payments for pass-through software costs. During the year ended December 31, 2024, the Company incurred costs of $20,191 pursuant to this work order. As of December 31, 2024, total costs of $20,191 have been incurred pursuant to this work order agreement.
The Company’s aggregate commitment pursuant to this clinical trial monitoring agreement, less amounts previously paid to date, totaled approximately $88,000 as of December 31, 2024, which is expected to be incurred through May 31, 2027.
Patent and License Agreements
National Institute of Health. Effective February 23, 2024, the Company entered into a Patent License Agreement (the “License Agreement”) with the National Institute of Neurological Disorders and Stroke (“NINDS”) and the National Cancer Institute (“NCI”), each an institute or center of the National Institute of Health (“NIH”). Pursuant to the License Agreement, the Company has licensed on an exclusive basis the NIH’s intellectual property rights claimed for a Cooperative Research and Development Agreement (“CRADA”) subject invention co-developed with the Company, and the licensed field of use, which focuses on promoting anti-cancer activity alone, or in combination with standard anti-cancer drugs. The scope of this clinical research extends to checkpoint inhibitors, immunotherapy, and radiation for the treatment of cancer. The License Agreement is effective, and shall extend, on a licensed product, licensed process, and country basis, until the expiration of the last-to-expire valid claim of the jointly owned licensed patent rights in each such country in the licensed territory, estimated at twenty years, unless sooner terminated.
The License Agreement contemplates that the Company will seek to work with pharmaceutical companies and clinical trial sites (including comprehensive cancer centers) to initiate clinical trials within timeframes that will meet certain benchmarks. Data from the clinical trials will be the subject of various regulatory filings for marketing approval in applicable countries in the licensed territories. Subject to the receipt of marketing approval, the Company would be expected to commercialize the licensed products in markets where regulatory approval has been obtained.
The Company is obligated to pay the NIH a non-creditable, non-refundable license issue royalty of $50,000 and a first minimum annual royalty within sixty days from the effective date of the Agreement. The first minimum annual royalty of $25,643 was prorated from the effective date of the License Agreement to the next subsequent January 1. Thereafter, the minimum annual royalty of $30,000 is due each January 1 and may be credited against any earned royalties due for sales made in that year. The license issue royalty of $50,000 and the first minimum annual royalty of $25,643, were paid in April 2024. The second minimum annual royalty for 2025 of $30,000, was paid in December 2024 and is included in other prepaid expenses at December 31, 2024 in the accompanying consolidated balance sheet.
| -73- |
The Company is obligated to pay the NIH, on a country-by-country basis, earned royalties of 2% on net sales of each royalty-bearing product and process, subject to reduction by 50% under certain circumstances relating to royalties paid by the Company to third parties, but not less than 1%. The Company’s obligation to pay earned royalties under the License Agreement commences on the date of the first commercial sale of a royalty-bearing product or process and expires on the date on which the last valid claim of the licensed product or licensed process expires in such country.
The Company is obligated to pay the NIH benchmark royalties, on a one-time basis, within sixty days from the first achievement of each such benchmark. The License Agreement defines four such benchmarks, which the Company is required to pursue based on “commercially reasonable efforts” as defined in the License Agreement, with deadlines of October 1, 2024, 2027, 2029 and 2031, respectively, each with a different specified benchmark payment amount payable within thirty days of achieving such benchmark. The October 1, 2024 benchmark of $100,000 was defined as the dosing of the first patient with a licensed product in a Phase 2 clinical study of such licensed product in the licensed fields of use. The Company had not commenced a Phase 2 clinical study as of December 31, 2024. The total of all such benchmark payments is $1,225,000.
The Company is obligated to provide annual reports to the NIH on its progress toward the development and commercialization of products under the licensed patents. These reports, due within sixty days following the end of each calendar year, must include updates on research and development activities, regulatory submissions, manufacturing efforts, sublicensing, and sales initiatives. If any deviations from the established commercial development plan or agreed-upon benchmarks occur, the Company is obligated to provide explanation and may amend the commercial development plan and the benchmarks, which, subject to certain conditions, the NIH shall not unreasonably withhold, condition, or delay approval of any request of the Company to amend the commercial development plan and/or the benchmarks and to extend the time periods of the benchmarks.
The Company is obligated to pay the NIH sublicensing royalties of 5% on sublicensing revenue received for granting each sublicense within sixty days of receipt of such sublicensing revenue.
During the year ended December 31, 2024, the Company incurred costs of $75,643 in connection with its obligations under the License Agreement. Such costs when incurred have been included in general and administrative costs in the Company’s consolidated statement of operations. As of December 31, 2024, total costs of $75,643 have been incurred pursuant to this agreement. The Company’s aggregate commitment pursuant to this agreement, less amounts previously paid to date, totaled approximately $1,795,000 as of December 31, 2024, which is expected to be incurred over approximately the next twenty years.
Moffitt. Effective August 20, 2018, the Company entered into an Exclusive License Agreement with Moffitt. Pursuant to the License Agreement, Moffitt granted the Company an exclusive license under certain patents owned by Moffitt (the “Licensed Patents”) relating to the treatment of MDS and a non-exclusive license under inventions, concepts, processes, information, data, know-how, research results, clinical data, and the like (other than the Licensed Patents) necessary or useful for the practice of any claim under the Licensed Patents or the use, development, manufacture or sale of any product for the treatment of MDS which would otherwise infringe a valid claim under the Licensed Patents.
On October 4, 2023, the Company received a counter-signed termination letter dated September 29, 2023 with respect to the Exclusive License Agreement dated August 20, 2018 between the Company and Moffitt, effective September 30, 2023. The Company and Moffitt agreed that no termination fee was due or payable by the Company, and Moffitt acknowledged that no payments are owed by the Company under the Agreement.
During the year ended December 31, 2023, the Company recorded a credit to operations of $9,109 representing the reversal of obligations previously recorded with respect to the Exclusive License Agreement.
| -74- |
Other Significant Agreements and Contracts
NDA Consulting Corp. On December 24, 2013, the Company entered into a consulting agreement with NDA Consulting Corp. for consultation and advice in the field of oncology research and drug development. As part of the consulting agreement, NDA also agreed to cause its president, Dr. Daniel D. Von Hoff, M.D., to serve on the Company’s Scientific Advisory Committee during the term of such consulting agreement. The term of the consulting agreement was for one year and provided for a quarterly cash fee of $4,000. The consulting agreement had been automatically renewed for additional one-year terms on its anniversary date, most recently on December 24, 2023, but was subsequently terminated effective September 30, 2024. Consulting and advisory fees charged to operations pursuant to this consulting agreement were $12,000 and $16,000 for the years ended December 31, 2024 and 2023, respectively
BioPharmaWorks. Effective September 14, 2015, the Company entered into a Collaboration Agreement with BioPharmaWorks, pursuant to which the Company engaged BioPharmaWorks to perform certain services for the Company. Those services included, among other things, assisting the Company to commercialize its products and strengthen its patent portfolio; identifying large pharmaceutical companies with a potential interest in the Company’s product pipeline; assisting in preparing technical presentations concerning the Company’s products; consultation in drug discovery and development; and identifying providers and overseeing tasks relating to clinical development of new compounds.
BioPharmaWorks was founded in 2015 by former Pfizer scientists with extensive multi-disciplinary research and development and drug development experience. The Collaboration Agreement was for an initial term of two years and automatically renews for subsequent annual periods unless terminated by a party not less than 60 days prior to the expiration of the applicable period. In connection with the Collaboration Agreement, the Company agreed to pay BioPharmaWorks a monthly fee of $10,000, subject to the right of the Company to pay a negotiated hourly rate in lieu of the monthly fee. Effective March 1, 2024, the compensation payable under the Collaboration Agreement was converted to an hourly rate structure.
The Company recorded charges to operations pursuant to this Collaboration Agreement of $39,200 and $120,000 during the years ended December 31, 2024 and 2023, respectively, which were included in research and development costs in the consolidated statements of operations.
Netherlands Cancer Institute. On October 8, 2021, the Company entered into a Development Collaboration Agreement with the Netherlands Cancer Institute, Amsterdam (“NKI”), one of the world’s leading comprehensive cancer centers, and Oncode Institute, Utrecht, a major independent cancer research center, for a term of three years. The Development Collaboration Agreement was subsequently modified by Amendment No. 1 thereto. The Development Collaboration Agreement is a preclinical study intended to identify the most promising drugs to be combined with LB-100, and potentially LB-100 analogues, to be used to treat a range of cancers, as well as to identify the specific molecular mechanisms underlying the identified combinations. The Company agreed to fund the preclinical study, at an approximate cost of 391,000 Euros and provide a sufficient supply of LB-100 to conduct the preclinical study.
On October 3, 2023, the Company entered into Amendment No. 2 to the Development Collaboration Agreement with NKI, which provides for additional research activities, extends the termination date of the Development Collaboration Agreement by two years to October 8, 2026, and added 500,000 Euros to the operating budget being funded by the Company.
On October 4, 2024, the Company entered into Amendment No. 3 to the Development Collaboration Agreement with NKI, which suspended Amendment No. 2 and provided for a new study term of one year and starts upon the dosing of the first patient in the clinical trial at a project cost of 100,000 Euros.
During the years ended December 31, 2024 and 2023, the Company incurred charges in the amount of $210,362 and $226,150, respectively, with respect to this agreement, which amounts are included in research and development costs in the Company’s consolidated statements of operations. As of December 31, 2024, total costs of $695,918 have been incurred pursuant to this agreement. The Company’s aggregate commitment pursuant to this agreement, less amounts previously paid to date, totaled approximately $104,000 as of December 31, 2024, which is expected to be incurred through October 8, 2026. As the work is being conducted in Europe and is paid for in Euros, final costs are subject to foreign currency fluctuations between the United States Dollar and the Euro.
| -75- |
MRI Global. As amended, the Company has contracted with MRI Global for stability analysis, storage and distribution of LB-100 for clinical trials in the United States. During the years ended December 31, 2024 and 2023, the Company incurred costs of $23,308 and $32,307, respectively, pursuant to this contract. As of December 31, 2024, total costs of $340,522 have been incurred pursuant to this contract.
The Company’s aggregate commitment pursuant to this contract, less amounts previously paid to date, totaled approximately $118,000 as of December 31, 2024.
Trends, Events and Uncertainties
Research and development of new pharmaceutical compounds is, by its nature, unpredictable. Although we will undertake research and development efforts with commercially reasonable diligence, there can be no assurance that our cash position will be sufficient to enable us to develop our pharmaceutical compounds to the extent needed to create future sales to sustain operations as contemplated herein.
There can be no assurance that our pharmaceutical compound will obtain the regulatory approvals and market acceptance to achieve sustainable revenues sufficient to support our operations. Even if we are able to generate revenues, there can be no assurance that we will be able to achieve operating profitability or positive operating cash flows. There can be no assurance that we will be able to secure additional financing, to the extent required, on acceptable terms or at all. If cash resources are insufficient to satisfy our ongoing cash requirements, we would be required to reduce or discontinue our research and development programs, or attempt to obtain funds, if available, through strategic alliances, joint ventures or other transaction structures that could require the Company to relinquish rights to and/or control of LB-100, or to discontinue operations entirely.
Other than as discussed above, we are not currently aware of any trends, events or uncertainties that are likely to have a material effect on our financial condition in the near term, although it is possible that new trends or events may develop in the future that could have a material effect on our financial condition.
ITEM 7A. QUANTITATIVE AND QUALITATIVE DISCLOSURES ABOUT MARKET RISK
Not applicable.
ITEM 8. FINANCIAL STATEMENTS AND SUPPLEMENTARY DATA
The Company’s consolidated financial statements and notes thereto and the related report of its independent registered public accounting firm are attached to this Annual Report on Form 10-K beginning on page F-1.
ITEM 9. CHANGES IN AND DISAGREEMENTS WITH ACCOUNTANTS ON ACCOUNTING AND FINANCIAL DISCLOSURE
Not applicable.
ITEM 9A. CONTROLS AND PROCEDURES
Evaluation of Disclosure Controls and Procedures
The Company’s management is responsible for establishing and maintaining a system of disclosure controls and procedures (as defined in Rule 13a-15(e) and 15d-15(e) under the Securities Exchange Act of 1934, as amended (the “Exchange Act”)) that is designed to ensure that information required to be disclosed by the Company in the reports that it files or submits under the Exchange Act is recorded, processed, summarized, and reported, within the time periods specified in the rules and forms. Disclosure controls and procedures include, without limitation, controls and procedures designed to ensure that information required to be disclosed by an issuer in the reports that it files or submits under the Exchange Act is accumulated and communicated to the issuer’s management, including its principal executive officer(s) and principal financial officer(s), or persons performing similar functions, as appropriate to allow timely decisions regarding required disclosure.
| -76- |
In accordance with Exchange Act Rules 13a-15 and 15d-15, an evaluation was completed under the supervision and with the participation of the Company’s management, including its Chief Executive Officer and its Chief Financial Officer, of the effectiveness of the design and operation of the Company’s disclosure controls and procedures as of the fiscal year ended December 31, 2023, the end of the most recent fiscal year covered by this report. Based on that evaluation, the Company’s management concluded that the Company’s disclosure controls and procedures were effective in providing reasonable assurance that information required to be disclosed in the Company’s reports filed or submitted under the Exchange Act was recorded, processed, summarized, and reported within the time periods specified in the rules and forms of the Securities and Exchange Commission (“SEC”).
Management’s Annual Report on Internal Control Over Financial Reporting
The Company’s management, including its Chief Executive Officer and its Chief Financial Officer, is responsible for establishing and maintaining adequate internal control over financial reporting (as defined in Rule 13a-15(f) and 15d-15(f) under the Exchange Act). Internal control over financial reporting is a process, including policies and procedures, designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external reporting purposes in accordance with U.S. generally accepted accounting principles. The Company’s internal control over financial reporting is designed to ensure that material information regarding the Company’s operations is made available to management and the Board of Directors to provide them reasonable assurance that the published financial statements are fairly presented.
The Company’s management assessed the Company’s internal control over financial reporting based on the Internal Control—Integrated Framework (2013 Framework) issued by the Committee of Sponsoring Organizations of the Treadway Commission (“COSO”). The Company’s system of internal control over financial reporting is designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with U.S. generally accepted accounting principles.
Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Therefore, even those systems determined to be effective can provide only reasonable assurance of achieving their control objectives. Furthermore, smaller reporting companies face additional limitations. Smaller reporting companies employ fewer individuals and can find it more difficult to properly segregate duties. Smaller reporting companies also tend to utilize general accounting software packages that lack a rigorous set of software controls.
A material weakness is a deficiency, or a combination of deficiencies, in internal control over financial reporting such that there is a reasonable possibility that a material misstatement of the Company’s annual or interim financial statements will not be prevented or deterred on a timely basis.
Based on the Company’s evaluation under the framework in COSO, the Company’s management, with the participation of its Chief Executive Officer and its Chief Financial Officer, concluded that the Company’s internal control over financial reporting was effective as of December 31, 2024.
Management believes that the consolidated financial statements included in this report fairly present, in all material respects, the Company’s financial condition, results of operations and cash flows as of and for the period ended December 31, 2024.
Auditor’s Report on Internal Control Over Financing Reporting
This report does not include an attestation report of the Company’s independent registered public accounting firm regarding internal control over financial reporting. Management’s report was not subject to attestation by the Company’s independent registered public accounting firm pursuant to rules of the SEC that permit the Company to provide only management’s report in this report.
| -77- |
Changes in Internal Control Over Financial Reporting
The Company’s management, including its Chief Executive Officer and its Chief Financial Officer, has determined that no change in the Company’s internal control over financial reporting (as that term is defined in Rules 13(a)-15(f) and 15(d)-15(f) of the Securities Exchange Act of 1934) occurred during or subsequent to the period ended December 31, 2024 that has materially affected, or is reasonably likely to materially affect, the Company’s internal control over financial reporting.
ITEM 9B. OTHER INFORMATION
Rule 10b5-1 Plans
During
the quarter ended December 31, 2024, no director or officer (as defined in Rule 16a-1(f) under the Exchange Act) of the Company
Insider Trading Policy
The Company has adopted insider trading policies and procedures governing the purchase, sale, and other disposition of its securities, which has been included as an exhibit to this report and has been posted to the investor information/governance section of the Company’s corporate website (www.lixte.com).
ITEM 9C. DISCLOSURE REGARDING FOREIGN JURISDICTIONS THAT PREVENT INSPECTIONS
Not applicable.
| -78- |
PART III
ITEM 10. DIRECTORS, EXECUTIVE OFFICERS AND CORPORATE GOVERNANCE
Directors and Executive Officers
The following table and text set forth the names of all of our directors and executive officers as of March 14, 2025. The Board of Directors is comprised of only one class. All of the directors will serve until the next annual meeting of stockholders and until their successors are elected and qualified, or until their earlier death, retirement, resignation or removal. The brief descriptions of the business experience of each director and executive officers and an indication of directorships held by each director in other companies subject to the reporting requirements under the Federal securities laws are provided herein below. Also provided are the biographies of the members of the Scientific Advisory Committee and our consultants.
Our directors and executive officers are as follows:
| Name | Age | Position(s) Held with Company | ||
| Bastiaan van der Baan | 53 | President, Chief Executive Officer, and Chairman of the Board of Directors | ||
| Dr. Jan H.M. Schellens | 68 | Consultant and Chief Medical Officer | ||
| Robert N. Weingarten | 72 | Vice President and Chief Financial Officer, Secretary | ||
| Dr. Stephen J. Forman | 76 | Director | ||
| Regina Brown | 61 | Director | ||
| Dr. Yun Yen | 70 | Director | ||
| Dr. René Bernards | 72 | Director |
Biographies of Directors and Executive Officers
Bastiaan van der Baan
Bastiaan (“Bas”) van der Baan was appointed to the Company’s Board of Directors effective June 17, 2022. Effective September 26, 2023, Mr. van der Baan replaced the Company’s founder, Dr. John S. Kovach, as President and Chief Executive Officer. Dr. Kovach passed away on October 5, 2023. Effective October 6, 2023, as a result of the passing of Dr. Kovach, Mr. van der Baan was appointed as Chairman of the Board of Directors.
Mr. van der Baan has over 20 years of experience in the biotechnology industry, with a key focus on oncology and diagnostics. He has extensive knowhow in the process of managing a compound from clinical development to reimbursement and commercialization, as well as the establishment of partnerships with the pharmaceutical industry, academic collaborators, distributors, insurance companies and governments to successfully launch new oncology products. Mr. van der Baan was most recently the Chief Clinical Officer of Agendia, an oncology molecular diagnostic company based in Irvine, California and Amsterdam, Netherlands through July 15, 2023. Mr. van der Baan is an independent director of Tethis S.p.A., a Milan, Italy-based developer of a novel platform for liquid biopsy testing. Mr. van der Baan was co-founder of ThromboDx, a liquid biopsy company that was acquired in 2016, Qameleon Therapeutics, a company developing synthetic lethal drug combinations for cancer treatment, and Oncosence, an oncology drug development company using senescence as target for drug development. Mr. van der Baan started his career in 1997 at a specialty chemicals division of Unilever that was acquired by ICI. In 2002, Mr. van der Baan joined Kreatech, a biotechnology company acquired by Leica that specialized in life science reagents for gene expression, DNA and protein analysis. Mr. van der Baan holds a Master’s Degree in Molecular Sciences from the Wageningen University in the Netherlands.
| -79- |
Dr. Jan H.M. Schellens, M.D., Ph.D.
Dr. Schellens was appointed as our Chief Medical Officer effective August 1, 2024. Dr. Schellens has more than 25 years of clinical experience as a medical oncologist, pharmacologist and clinical pharmacologist, including more than two decades developing and bringing new drugs to market. Co-author of more than 900 publications in peer-reviewed scientific journals, Dr. Schellens has held leadership positions at the Netherlands Cancer Institute in Amsterdam and the Dr. Daniel den Hoed Clinic-Erasmus University in Rotterdam. He was professor of clinical pharmacology at Utrecht University in the Netherlands, where he earned his M.D. degree, and he served as a board member and Chief Medical Officer of Byondis B.V. from January 2019 through September 2023. He also earned a Ph.D. degree in Pharmaceutical Sciences from Leiden University in Leiden, Netherlands. Dr. Schellens served for 17 years as a board member of the Dutch Medicines Evaluation Board and for 12 years as a member and chairperson of the Scientific Advisory Board Oncology of the EMA. From 2016 to the present, he has served as a part-time Chief Medical Officer of Modra Pharmaceuticals B.V., an Amsterdam-based company that successfully completed a Phase 2b clinical study of ModraDoc006/r, a boosted oral taxane therapeutic, in contrast to the standard-of-care IV chemotherapy docetaxel, in patients with prostate cancer.
Dr. Schellens plays a leadership role in the planning, implementation and oversight of the Company’s clinical trials and is responsible for assisting in the development of strategic clinical goals and the implementation and safety monitoring of investigational studies. Dr. Schellens is the primary medical monitor for all clinical investigational studies, and for the oversight of third party CRO monitors. He is responsible for the regulatory strategy and implementation of the strategy and the primary contact for regulators. Dr. Schellens works closely with the Company’s Chief Executive Officer on the development of strategic goals needed to ensure the timely implementation of appropriate clinical studies needed for the successful registration of therapeutics products. Dr. Schellens services are principally rendered in the Netherlands.
Robert N. Weingarten
Mr. Weingarten was appointed to serve as our Vice President and Chief Financial Officer effective August 12, 2020. Mr. Weingarten is an experienced business consultant and advisor with a consulting practice focusing on accounting and SEC compliance issues. Mr. Weingarten was familiar with the financial and business operations of the Company, as he had provided accounting and financial consulting services to the Company for a number of years prior to his appointment as Vice President and Chief Financial Officer with respect to the preparation of the Company’s consolidated financial statements and certain other financial and compliance matters.
Since 1979, Mr. Weingarten has provided such financial consulting and advisory services, has acted as chief financial officer, and has served on the boards of directors of numerous public companies in various stages of development, operation or reorganization. Mr. Weingarten has experience in a variety of industries, including the pharmaceutical industry.
Mr. Weingarten was a Director of Guardion Health Sciences, Inc. since June 2015 and was Chairman of its Board of Directors from July 2020 through October 2024. Mr. Weingarten also served on the audit, compensation, and nominating and corporate governance committees of Guardion Health Sciences, Inc. during such period. Previously, Mr. Weingarten served as Lead Director on Guardion’s Board of Directors from January 2017 through March 2020. Mr. Weingarten received a B.A. in Accounting from the University of Washington in 1974, an M.B.A. in Finance from the University of Southern California in 1975, and is a Certified Public Accountant (inactive) in the State of California.
Dr. Stephen J. Forman
Stephen J. Forman, M.D., was appointed to our Board of Directors effective May 13, 2016. Dr. Forman is an internationally recognized expert in hematologic malignancies and bone marrow transplantation, and is a leader in preclinical and clinical cancer research. Dr. Forman was appointed to our Board of Directors on May 13, 2016. He is co-editor of Thomas’ Hematopoietic Cell Transplantation, a definitive textbook for clinicians, scientists and health care professionals. Dr. Forman is the Francis and Kathleen McNamara Distinguished Chair in Hematology and Hematopoietic Cell Transplantation at the City of Hope Comprehensive Cancer Center, a position he has held since 1987.
In nearly 40 years at the City of Hope, Dr. Forman has been instrumental in advancing the survival rates for patients suffering from cancers of the blood and immune system such as leukemia, lymphoma and myeloma.
As Director of the T Cell Immunotherapy Research Laboratory, his current research is focused on cancer immunotherapy, using the body’s own immune system to attack cancer. Pharmacological enhancement of patients’ immune responses to their cancers is of special interest to the Company, as the enzyme target of its lead clinical compound, LB-100, has been reported to be critical to immune function. Much of Dr. Forman’s current work centers on T-cells and their cancer-fighting potential.
| -80- |
Dr. Yun Yen
Yun Yen, M.D., Ph.D., F.A.C.P., was appointed to our Board of Directors effective August 4, 2018. Dr. Yen is a physician, scientist, innovator, and philanthropist. Dr. Yen was appointed to our Board of Directors on August 4, 2018. He is widely regarded as an expert in ribonucleotide reductase, a critical target in cancer therapy and diagnostics. He is President Emeritus of Taipei Medical University (TMU) and Chair Professor of the Ph.D. Program for Cancer Biology and Drug Discovery. Prior to TMU, Dr. Yen was the Allen and Lee Chao Endowed Chair in Developmental Cancer Therapeutics, Chair of Molecular Pharmacology Department, Associate Director for Translational Research, and Co-Director of the Developmental Cancer Therapeutics Program at the City of Hope NCI-designated Comprehensive Cancer Center, Duarte California. He has published more than 300 peer-reviewed articles, holds over 60 patents, and has commercialized multiple methodologies involving nanoparticles, small and large molecule drugs, biomarkers, stem cells, and medical devices. Dr. Yen has also founded philanthropic organizations aimed at serving the global cancer community and holds membership in numerous professional societies. He serves on the boards of Fulgent Genetics and Tanvex BioPharma Inc.
Regina Brown, CPA
Regina Brown was appointed to our Board of Directors effective May 11, 2021. Ms. Brown has been a practicing accountant for over thirty years. Her practice has a wide range of clients, varying in size, industry and geographic locations, including large national corporations listed on the New York Stock Exchange, as well as Southern California businesses. Other clients consist of professionals, wholesalers and high net worth individuals. Many of her clients have international and cross-border operations.
As a consequence of her depth of experience, she regularly assists other professionals with their client’s issues and performs tax research and analysis in connection with litigation and other matters, including marital dissolution, tax and accounting with respect to mergers and acquisitions, implementation of internal controls, and extensive work in the area of trusts and estates. International tax matters and compliance are also a significant part of her practice. Ms. Brown is a member in good standing of the California Society of CPAs and the American Institute of Certified Public Accountants and has appeared as a speaker before both organizations.
Dr. René Bernards
Dr. René Bernards was appointed to our Board of Directors effective June 15, 2022. Dr. Bernards is a leader in the field of molecular carcinogenesis, working at the Netherlands Cancer Institute in Amsterdam. His research focuses on identifying effective new drug combinations, new drug targets, and mechanisms of resistance to anti-cancer drugs. He has also co-founded four biotechnology companies to bring his scientific discoveries to clinical oncology practice. He is a member of the Royal Netherlands Academy of Sciences, an International Honorary Member of the American Academy of Arts and Sciences and an International Member of the National Academy of Sciences (USA). Additionally, he is a fellow of the American Association for Cancer Research (AACR). Dr. Bernards has presented new data on the unexpected effectiveness of the Company’s lead clinical compound, LB-100, when given with a variety of standard and investigational anti-cancer compounds that have only modest activity on their own.
Family Relationships
Eric Forman, the Company’s Vice President and Chief Operating Officer during the years ended December 31, 2024, 2023 and 2022 was the son of board member Dr. Stephen Forman and the son-in-law of former board member Gil Schwartzberg, who passed away on October 30, 2022. Julie Forman, the wife of Eric Forman and the daughter of the late Gil Schwartzberg, is Vice President of Morgan Stanley Wealth Management, where the Company’s cash is deposited and managed, and the Company maintains a continuing banking relationship. Eric Forman resigned as Vice President and Chief Operating Officer of the Company effective December 31, 2024.
| -81- |
Committees of Our Board of Directors
Our Board of Directors directs the management of our business and affairs, as provided by Delaware law, and conducts its business through meetings of the Board of Directors and its standing committees. We have a standing audit committee and compensation committee. The Board of Directors serves in place of a nominating and corporate governance committee. In addition, from time to time, special committees may be established under the direction of the Board of Directors when necessary to address specific issues.
Audit Committee
Our audit committee is responsible for, among other things:
| ● | approving and retaining the independent auditors to conduct the annual audit of our financial statements; | |
| ● | reviewing the proposed scope and results of the audit; | |
| ● | reviewing and pre-approving audit and non-audit fees and services; |
| ● | reviewing accounting and financial controls with the independent auditors and our financial and accounting staff; | |
| ● | reviewing and approving transactions between us and our directors, officers and affiliates; | |
| ● | establishing procedures for complaints received by us regarding accounting matters; | |
| ● | overseeing internal audit functions, if any; and | |
| ● | preparing the report of the audit committee that the rules of the SEC require to be included in our annual meeting proxy statement. |
Our audit committee currently consists of Regina Brown, Dr. Yun Yen and Dr. René Bernards, with Ms. Brown serving as chair. Our Board of Directors has determined that each of the committee members meet the definition of an “independent director,” as defined under Nasdaq rules, and that they each meet the independence standards under Rule 10A-3 of the Exchange Act. Each member of our audit committee meets the financial literacy requirements of the Nasdaq rules. In addition, our Board of Directors has determined that Ms. Brown qualifies as an “audit committee financial expert,” as such term is defined in Item 407(d)(5) of Regulation S-K. Our Board of Directors has adopted a written charter for the audit committee, which is available on our corporate website at www.lixte.com.
Compensation Committee
Our compensation committee is responsible for, among other things:
| ● | reviewing and recommending the compensation arrangements for executive management; | |
| ● | establishing and reviewing general compensation policies with the objective to attract and retain superior talent, to reward individual performance and to achieve our financial goals; | |
| ● | administering our stock incentive plans; and | |
| ● | preparing the report of the compensation committee that the rules of the SEC require to be included in our annual meeting proxy statement. |
Our compensation committee currently consists of Dr. Yun Yen, Regina Brown and Dr. René Bernards, with Dr. Yen serving as chair. Our Board of Directors has determined that each of the three committee members meet the definition of an “independent director”, as defined under Nasdaq rules. Our Board of Directors has adopted a written charter for the compensation committee, which is available on our corporate website at www.lixte.com.
| -82- |
Nominating and Corporate Governance
Although our Board of Directors serves in place of a nominating and corporate governance committee, our independent directors on the Board of Directors are responsible for, among other things:
| ● | nominating members of the Board of Directors; | |
| ● | developing a set of corporate governance principles applicable to the Company; and | |
| ● | overseeing the evaluation of our Board of Directors. |
Our Board of Directors may adopt resolutions addressing, among other things, the nomination process, as may be necessary in the future.
Code of Ethics
Our Board of Directors has adopted a code of ethics covering all of our executive officers and key employees. A copy of our code of ethics will be furnished without charge to any person upon written request. Requests should be sent to: Secretary, Lixte Biotechnology Holdings, Inc., 680 East Colorado Boulevard, Suite 180, Pasadena, California 91101.
Limitations on Liability and Indemnification Matters
Our Certificate of Incorporation contains provisions that limit the liability of our current and former directors for monetary damages to the fullest extent permitted by Delaware law. Delaware law provides that directors of a corporation will not be personally liable for monetary damages for any breach of fiduciary duties as directors, except liability for:
| ● | any breach of the director’s duty of loyalty to the corporation or its stockholders; | |
| ● | any act or omission not in good faith or that involves intentional misconduct or a knowing violation of law; | |
| ● | unlawful payments of dividends or unlawful stock repurchases or redemptions as provided in Section 174 of the Delaware General Corporation Law; or | |
| ● | any transaction from which the director derived an improper personal benefit. |
This limitation of liability does not apply to liabilities arising under federal securities laws and does not affect the availability of equitable remedies such as injunctive relief or rescission.
Our Certificate of Incorporation provides that we are authorized to indemnify our directors and officers to the fullest extent permitted by Delaware law. Our Amended and Restated Bylaws provide that we are required to indemnify our directors and executive officers to the fullest extent permitted by Delaware law. Our Amended and Restated Bylaws also provide that, upon satisfaction of certain conditions, we are required to advance expenses incurred by a director or executive officer in advance of the final disposition of any action or proceeding, and permit us to secure insurance on behalf of any officer, director, employee or other agent for any liability arising out of their actions in that capacity, regardless of whether we would otherwise be permitted to indemnify them under the provisions of Delaware law. Our Amended and Restated Bylaws also provide our Board of Directors with discretion to indemnify our other officers and employees when determined appropriate by our Board of Directors. We have entered into agreements to indemnify our directors, executive officers and other employees as determined by the Board of Directors. With certain exceptions, these agreements provide for indemnification for related expenses, including, among other things, attorneys’ fees, judgments, fines and settlement amounts incurred by any of these individuals in any action or proceeding. We believe that these provisions and agreements are necessary to attract and retain qualified persons as directors and officers. We have obtained customary directors and officers liability insurance.
| -83- |
The limitation of liability and indemnification provisions in our Certificate of Incorporation and Amended and Restated Bylaws may discourage stockholders from bringing a lawsuit against our directors for an alleged breach of their fiduciary duty. These provisions may also reduce the likelihood of derivative litigation against our directors and officers, even though an action, if successful, might benefit us and other stockholders. Furthermore, a stockholder’s investment may be adversely affected to the extent that we pay the costs of settlement and damage awards against directors and officers as required by these indemnification provisions. At present, there is no pending litigation or proceeding involving any of our directors, officers or employees for which indemnification is sought, and we are not aware of any threatened litigation that may result in claims for indemnification.
Compliance with Section 16(a) of the Securities Exchange Act of 1934, as Amended
Section 16(a) of the Securities Exchange Act of 1934, as amended, requires the Company’s directors and executive officers and persons who own more than 10% of a registered class of the Company’s equity securities to file various reports with the Securities and Exchange Commission concerning their holdings of, and transactions in, securities of the Company. Copies of these filings are required to be furnished to the Company.
To the Company’s knowledge, based solely on its review of the copies of the Section 16(a) reports furnished to the Company and any written representations to the Company that no other reports were required, the Company believes that all individual filing requirements applicable to a director, officer, or beneficial owner of more than 10% of the Company’s common stock were complied with under Section 16(a) of the Exchange Act during the year ended December 31, 2023, except as follows: Rene Bernards was late in filing his Form 4 in connection with the grant of stock options on June 30, 2024, and Rene Bernards, Yun Yen, Regina Brown and Stephen Forman were late in filing their Form 4’s in connection with the grant of stock options on September 30, 2024.
ITEM 11. EXECUTIVE COMPENSATION
OFFICER AND DIRECTOR COMPENSATION
The table set forth below presents the compensation awarded to, earned by, or paid to our named executive officers for the years ended December 31, 2024, 2023 and 2022.
OFFICER COMPENSATION TABLE
| Executive | Year | Salary ($) |
Bonus ($) |
Stock
Awards ($) |
Option
Awards ($)(1) |
Non-Equity
Incentive Plan Compensation ($) |
Non-Qualified
Deferred Compensation Earnings ($) |
All
Other Compensation ($) |
Total ($) |
||||||||||||||||||||||||||
| Bas van der Baan (6) | 2024 | 153,495 | - | - | - | - | - | - | 153,495 | ||||||||||||||||||||||||||
| 2023 | 40,639 | - | - | 403,066 | - | - | - | 443,705 | |||||||||||||||||||||||||||
| 2022 | - | - | - | - | - | - | - | - | |||||||||||||||||||||||||||
| John S. Kovach (2) | 2024 | - | - | - | - | - | - | - | - | ||||||||||||||||||||||||||
| 2023 | 190,860 | - | - | - | - | - | - | 190,860 | |||||||||||||||||||||||||||
| 2022 | 250,000 | - | - | 65,640 | - | - | - | 315,640 | |||||||||||||||||||||||||||
| James S. Miser (3) | 2024 | 102,083 | - | - | - | - | - | - | 102,083 | ||||||||||||||||||||||||||
| 2023 | 175,000 | - | - | - | - | - | - | 175,000 | |||||||||||||||||||||||||||
| 2022 | 175,000 | - | - | 65,640 | - | - | - | 240,640 | |||||||||||||||||||||||||||
| Robert N. Weingarten (4) | 2024 | 175,000 | - | - | - | - | - | - | 175,000 | ||||||||||||||||||||||||||
| 2023 | 175,000 | - | - | - | - | - | - | 175,000 | |||||||||||||||||||||||||||
| 2022 | 175,000 | - | - | 65,640 | - | - | - | 240,640 | |||||||||||||||||||||||||||
| Eric J. Forman (5) | 2024 | 200,000 | - | - | - | - | - | - | 200,000 | ||||||||||||||||||||||||||
| 2023 | 200,000 | - | - | - | - | - | - | 200,000 | |||||||||||||||||||||||||||
| 2022 | 178,819 | - | - | 65,640 | - | - | - | 244,459 | |||||||||||||||||||||||||||
| Jan H.M. Schellens (7) | 2024 | 56,226 | - | - | 29,074 | - | - | - | 85,300 | ||||||||||||||||||||||||||
| 2023 | - | - | - | - | - | - | - | - | |||||||||||||||||||||||||||
| 2022 | - | - | - | - | - | - | - | - | |||||||||||||||||||||||||||
(1) Consists of grant date fair value of option award calculated pursuant to the Black-Scholes option-pricing model.
| -84- |
(2) John S. Kovach was the President and Chief Executive Officer from inception through September 26, 2023. Effective July 15, 2020, the Company entered into an employment agreement with Dr. Kovach. On November 6, 2022, Dr. Kovach was awarded an option grant for 20,000 shares of common stock, exercisable for a period of five years at $20.00 per share and valued at $3.282 per share. The employment agreement with Dr. Kovach terminated upon his death on October 5, 2023.
(3) James S. Miser was appointed as Chief Medical Officer on August 1, 2020. In connection with his employment agreement, Dr. Miser was awarded an option grant for 8,334 shares of common stock, exercisable for a period of five years at $71.40 per share and valued at $68.718 per share. On November 6, 2022, Dr. Miser was awarded an option grant for 20,000 shares of common stock, exercisable for a period of five years at $20.00 per share and valued at $3.282 per share. On May 29, 2024, the Company elected not to renew its employment agreement with Dr. Miser, as a result of which such employment agreement expired on July 31, 2024.
(4) Robert N. Weingarten was appointed as Vice President and Chief Financial Officer on August 12, 2020. In connection with his employment agreement, Mr. Weingarten was awarded an option grant for 5,833 shares of common stock, exercisable for a period of five years at $71.40 per share and valued at $68.718 per share. On November 6, 2022, Mr. Weingarten was awarded an option grant for 20,000 shares of common stock, exercisable for a period of five years at $20.00 per share and valued at $3.282 per share.
(5) Eric J. Forman was Chief Administrative Officer from July 15, 2020 through November 6, 2020. In connection with his employment agreement, Mr. Forman was awarded an option grant for 5,833 shares of common stock, exercisable for a period of five years at $71.40 per share and valued at $68.718 per share. Effective November 6, 2022, Mr. Forman was appointed as Vice President and Chief Operating Officer. On November 6, 2022, Mr. Forman was awarded an option grant for 20,000 shares of common stock, exercisable for a period of five years at $20.00 per share and valued at $3.282 per share. The employment agreement with Mr. Forman terminated upon his resignation as an officer of the Company effective December 31, 2024.
(6) Bas van der Baan was appointed as President and Chief Executive Officer on September 26, 2023. In connection with his employment agreement, Mr. van der Baan was awarded an option grant for 250,000 shares of common stock exercisable for a period of five years at $1.95 per share and valued at $1.612 per share. The compensation information provided herein excludes compensation as a Director received before his appointment as President and Chief Executive Officer.
(7) On May 31, 2024, the Company entered into a consulting agreement with Dr. Jan H.M. Schellens, M.D., Ph.D., Pursuant to the agreement, effective July 1, 2024, the Company engaged Dr. Schellens as a consultant, and, effective August 1, 2024, as the Company’s Chief Medical Officer. In connection with his employment agreement, Mr. Schellens was awarded an option grant for 15,000 shares of common stock exercisable for a period of five years at $2.39 per share and valued at $1.938 per share.
There were no option exercises by officers during the years ended December 31, 2024, 2023 or 2022.
| -85- |
Outstanding Equity Awards at December 31, 2024
The table set forth below presents information regarding outstanding stock options held by our named executive officers as of December 31, 2024.
| NAME |
GRANT DATE |
VESTING COMMENCEMENT DATE |
NUMBER OF SECURITIES UNDERLYING UNEXERCISED OPTIONS EXERCISABLE (#) |
NUMBER OF SECURITIES UNDERLYING UNEXERCISED OPTIONS UNEXERCISABLE (#) |
OPTION EXERCISE PRICE ($) |
OPTION EXPIRATION DATE |
||||||||||||
| Bas van der Baan | June 17, 2022 (1) | June 17, 2022 | 25,000 | - | 7.40 | June 17, 2027 | ||||||||||||
| June 30, 2023 (1) | September 30, 2023 | 7,500 | 2,500 | 5.88 | June 30, 2028 | |||||||||||||
| September 26, 2023 | December 31, 2023 | 104,165 | 145,835 | 1.95 | September 26, 2028 | |||||||||||||
| Dr. Jan H.M. Schellens | July 1, 2024 | September 30, 2024 | 2,500 | 12,500 | 2.39 | July 1, 2029 | ||||||||||||
| Dr. James S. Miser | August 1, 2020 | August 1, 2020 | 8,334 | - | 71.40 | July 31, 2025 | ||||||||||||
| November 6, 2022 | November 6, 2022 | 10,000 | - | 20.00 | July 31, 2025 | |||||||||||||
| Robert N. Weingarten | August 12, 2020 | August 12, 2020 | 5,833 | - | 71.40 | August 12, 2025 | ||||||||||||
| November 6, 2022 | November 6, 2022 | 15,000 | 5,000 | 20.00 | November 6, 2027 | |||||||||||||
| Eric J. Forman | August 12, 2020 | August 12, 2020 | 5,833 | - | 71.40 | August 12, 2025 | ||||||||||||
| November 6, 2022 | November 6, 2022 | 15,000 | - | 20.00 | December 31, 2025 | |||||||||||||
(1) Granted in his capacity as a Director before date of officer appointment on September 26, 2023.
Based on a fair market value of $2.03 per share on December 31, 2024, the intrinsic value attributed to exercisable but unexercised common stock options held by our named executive officers was approximately $8,000 at December 31, 2024.
Employment Agreements; Compensation
During July and August 2020, the Company entered into one-year employment agreements with its executive officers, consisting of Dr. John S. Kovach, Eric J. Forman, Dr. James S. Miser, and Robert N. Weingarten, payable monthly, as described below. The employment agreements were automatically renewable for additional one-year periods unless terminated by either party upon 60 days written notice prior to the end of the applicable one-year period, or by death, or by termination for cause. These employment agreements were automatically renewed for additional one-year periods in July and August 2021, 2022, 2023 and 2024.
Dr. John Kovach. On July 15, 2020, the Company entered into an employment agreement with Dr. John Kovach to continue to act as the Company’s President, Chief Executive Officer and Chief Scientific Officer, with an annual salary of $250,000, payable monthly. His responsibilities included the oversight of the Company’s entire operations and strategic planning, and to act as the primary contact between the Company’s executive team and the Board of Directors, to whom he reported. Dr. Kovach supervised all scientific endeavors, providing guidance to the Chief Medical Officer. He was the principal spokesperson for the Company. The effective date of the agreement was October 1, 2020 and remained in effect until the earlier of (i) one year from the effective date, automatically renewable for additional one-year periods unless terminated by either party upon 60 days written notice prior to the end of the applicable one-year period, (ii) his death, or (iii) termination for cause. The employment agreement with Dr. Kovach terminated upon his death on October 5, 2023.
| -86- |
Eric Forman. On July 15, 2020, as amended on August 12, 2020, the Company entered into an employment agreement with Eric Forman, to act as the Company’s Chief Administrative Officer, reporting directly to the Company’s Chief Executive Officer, with an annual salary of $120,000, payable monthly. Effective May 1, 2021, Mr. Forman’s annual salary was increased to $175,000. Effective November 6, 2022, Mr. Forman was promoted to Vice President and Chief Operating Officer, with an annual salary of $200,000. Mr. Forman’s primary function was to oversee the Company’s internal operations, including IT, licensing, legal, personnel, marketing, and corporate governance. Mr. Forman was also granted stock options to acquire 5,833 shares of the Company’s common stock. The effective date of the employment agreement was October 1, 2020 and remained in effect until the earlier of (i) one year from the effective date, automatically renewable for additional one-year periods unless terminated by either party upon 60 days written notice prior to the end of the applicable one-year period, (ii) his death, or (iii) termination for cause. The employment agreement with Mr. Forman terminated upon his resignation as an officer of the Company effective December 31, 2024.
Dr. James Miser. On August 1, 2020, the Company entered into an employment agreement with Dr. James Miser, M.D., pursuant to which Dr. Miser was appointed as the Company’s Chief Medical Officer, with an annual salary of $150,000. Effective May 1, 2021, Dr. Miser’s annual salary was increased to $175,000. Dr. Miser was required to devote at least 50% of his business time to the Company’s activities. Dr. Miser was also granted stock options to acquire 8,334 shares of the Company’s common stock. The effective date of the agreement was August 1, 2020 and remained in effect until the earlier of (i) one year from the effective date, automatically renewable for additional one-year periods unless terminated by either party upon 60 days written notice prior to the end of the applicable one-year period, (ii) his death, or (iii) termination for cause. On May 29, 2024, the Company elected not to renew its employment agreement with Dr. Miser, as a result of which such employment agreement expired on July 31, 2024.
Dr. Jan H.M. Schellens, M.D., Ph.D. On May 31, 2024, the Company entered into a consulting agreement with Dr. Jan H.M. Schellens, M.D., Ph.D. Pursuant to the agreement, effective July 1, 2024, the Company engaged Dr. Schellens as a consultant, and, effective August 1, 2024, as the Company’s Chief Medical Officer. The term of the agreement is in effect from July 1, 2024 until the earliest of (i) termination by either party upon sixty days’ notice, (ii) Dr. Schellens’ death or disability, or (iii) termination by the Company for breach as provided in the agreement. Under the agreement, Dr. Schellens provides his services for two days per week with the specific days in each week based on arrangements agreed to from time to time between Dr. Schellens and the Company’s Chief Executive Officer. The Company pays Dr. Schellens an annual compensation of 104,000 Euros (approximately $108,000 as of December 31, 2024), payable on a monthly basis. On July 1, 2024, in connection with the consulting agreement, Dr. Schellens was granted stock options to purchase 15,000 shares of the Company’s common stock.
Robert N. Weingarten. On August 12, 2020, the Company entered into an employment agreement with Robert N. Weingarten pursuant to which Mr. Weingarten was appointed as the Company’s Vice-President and Chief Financial Officer, with an annual salary of $120,000. Effective May 1, 2021, Mr. Weingarten’s annual salary was increased to $175,000. Mr. Weingarten was also granted stock options to acquire 5,833 shares of the Company’s common stock. The effective date of the agreement was August 12, 2020 and remained in effect until the earlier of (i) one year from the effective date, automatically renewable for additional one-year periods unless terminated by either party upon 60 days written notice prior to the end of the applicable one-year period, (ii) his death, or (iii) termination for cause.
Bas van der Baan. Effective September 26, 2023, the Company entered into an employment agreement with Bas van der Baan to act as the Company’s President and Chief Executive Officer and as Vice Chairman of the Board of Directors, with an annual salary of $150,000. Effective October 6, 2023, Mr. van der Baan was appointed as Chairman of the Board of Directors upon the death of Dr. Kovach on October 5, 2023. Mr. van der Baan’s annual salary may be increased from time to time at the sole discretion of the Board of Directors. In addition, Mr. van der Baan will be eligible to receive an annual bonus as determined at the sole discretion of the Board of Directors. Mr. van der Baan was also granted stock options to acquire 250,000 shares of the Company’s common stock. The term of the employment agreement is for three years and is automatically renewable for additional one-year periods unless terminated by either party, subject to early termination provisions as described in the employment agreement.
Policies and Practices – Option Grants
Directors. The Company has a comprehensive compensation program for its non-officer directors for their service on the Board of Directors. This program, as amended, has been in place since April 9, 2021. The Company, with the input and advice of its Compensation Committee, has issued only stock options to its officers and directors.
| -87- |
Equity compensation for directors under this compensation program is as follows:
Appointment of new directors – The Company grants options to purchase 25,000 shares of common stock, exercisable for a period of five years, at the closing market price on the date of grant, vesting 50% on the grant date and the remaining 50% vesting 12.5% on the last day of each calendar quarter beginning in the quarter immediately subsequent to the date of the grant until fully vested, subject to continued service. At the discretion of the Board of Directors, for a nominee to the Board of Directors who is restricted by their respective institution or employer from receiving equity-based compensation, in lieu of the grant of such stock options, the Company may elect to pay a one-time cash fee of $100,000 to such director, payable upfront.
Annual grant of options to directors – Effective on the last business day of the month of June, the Company grants options to purchase 10,000 shares of common stock, exercisable for a period of five years, at the closing market price on the date of grant, vesting 12.5% on the last day of each calendar quarter beginning in the quarter immediately subsequent to the date of grant until fully vested, subject to continued service. If any director has served for less than 12 full calendar months on the grant date, the amount of such stock option grant is prorated based on the length of service of such director. At the discretion of the Board of Directors, for a nominee to the Board of Directors who is restricted by their respective institution or employer from receiving equity-based compensation, in lieu of the grant of such stock options, the Company may elect to pay an annual cash fee of $40,000 to such director, payable quarterly.
Officers. The Company has no specific policy or program with respect to the discretionary grant of options to its officers. The Company granted options to its officers concurrent with their respective appointments during the year ended December 31, 2020. The Company also granted discretionary stock options to its officers during the year ended December 31, 2022. It is the Company’s policy that any such option grants take into account the existence of material non-public information when determining the timing of such a grant and the specific terms of such award.
Compensation Clawback Policy
The Board of Directors believes that it is in the best interests of the Company and its stockholders to create and maintain a culture that emphasizes integrity and accountability and that reinforces the Company’s pay-for-performance compensation philosophy. The Board of Directors has therefore adopted a compensation recoupment policy, which provides for the recovery of erroneously awarded incentive compensation from the Company’s executive officers in the event of a triggering event, and which has been filed as an exhibit to this report and has been posted to the investor information/governance section of the Company’s corporate website (www.lixte.com).
Board of Directors Compensation
On May 11, 2021, the Board of Directors appointed Regina Brown to the Board of Directors. In connection with her appointment to the Board of Directors, and in accordance with the Company’s cash and equity compensation package for members of the Board of Directors, Ms. Brown was granted stock options to purchase 25,000 shares of the Company’s common stock, exercisable for a period of five years at an exercise price of $28.00 per share (the closing market price on the grant date), vesting 50% on the grant date and the remainder vesting 12.5% on the last day of each subsequent calendar quarter-end until fully vested, subject to continued service. The fair value of these stock options, as calculated pursuant to the Black-Scholes option-pricing model, was determined to be $658,363 ($2.6335 per share), of which $329,188 was attributable to the portion of the stock options fully vested on May 11, 2021 and was therefore charged to operations on that date. The remaining unvested portion of the fair value of the stock options was charged to operations ratably from May 11, 2021 through June 30, 2023. During the years ended December 31, 2023, 2022 and 2021, the Company recorded charges to general and administrative costs in the consolidated statement of operations of $76,388, $154,042 and $427,944, respectively, with respect to these stock options.
On June 30, 2021, the Board of Directors, in accordance with the Company’s cash and equity compensation package for members of the Board of Directors, granted to each of the five non-officer directors of the Company stock options to purchase 10,000 shares (a total of 50,000 shares) of the Company’s common stock, exercisable for a period of five years at an exercise price of $30.30 per share (the closing market price on the grant date), vesting 12.5% on the last day of each subsequent calendar quarter-end until fully vested, subject to continued service. The fair value of these stock options, as calculated pursuant to the Black-Scholes option-pricing model, was determined to be $1,421,095 ($28.4225 per share), which was charged to operations ratably from July 1, 2021 through June 30, 2023. During the years ended December 31, 2023, 2022 and 2021, the Company recorded charges to general and administrative costs in the consolidated statement of operations of $211,413, $638,915 and $358,200, respectively, with respect to these stock options.
| -88- |
Effective as of June 15, 2022, Dr. René Bernards was appointed to the Company’s Board of Directors. As a new director, in lieu of a grant of stock options, Dr. Bernards received a one-time cash board fee of $100,000, payable immediately, and an annual cash board fee of $40,000, payable quarterly. During the years ended December 31, 2023 and 2022, the Company recorded charges to general and administrative costs in the consolidated statement of operations of $62,500 and $133,873, respectively, with respect to his cash board compensation.
On June 17, 2022, the Board of Directors appointed Bas van der Baan to the Board of Directors. In connection with his appointment to the Board of Directors, and in accordance with the Company’s cash and equity compensation package for members of the Board of Directors, Mr. Baan was granted stock options to purchase 25,000 shares of the Company’s common stock, exercisable for a period of five years at an exercise price of $7.40 per share (the closing market price on the grant date), vesting 50% on the grant date and the remainder vesting 12.5% on the last day of each subsequent calendar quarter-end until fully vested, subject to continued service. The fair value of these stock options, as calculated pursuant to the Black-Scholes option-pricing model, was determined to be $158,525 ($6.341 per share), of which $79,263 was attributable to the portion of the stock options fully vested on June 17, 2022 and was therefore charged to operations on that date. The remaining unvested portion of the fair value of the stock options is being charged to operations ratably from June 17, 2022 through June 30, 2024. During the years ended December 31, 2023 and 2022, the Company recorded charges to general and administrative costs in the consolidated statement of operations of $38,885 and $100,249, respectively, with respect to these stock options.
On June 30, 2022, the Board of Directors, in accordance with the Company’s cash and equity compensation package for members of the Board of Directors, granted to each of the five non-officer directors of the Company stock options to purchase 10,000 shares (a total of 50,000 shares) of the Company’s common stock, exercisable for a period of five years at an exercise price of $7.40 per share (the closing market price on the grant date), vesting 12.5% on the last day of each subsequent calendar quarter-end until fully vested, subject to continued service. The fair value of these stock options, as calculated pursuant to the Black-Scholes option-pricing model, was determined to be $316,700 ($6.334 per share), which is being charged to operations ratably from July 1, 2022 through June 30, 2024. For the years ended December 31, 2023 and 2022, the Company recorded charges to general and administrative costs in the consolidated statement of operations of $94,881 and $63,777, respectively, with respect to these stock options.
On June 30, 2023, the Board of Directors, in accordance with the Company’s cash and equity compensation package for members of the Board of Directors, granted to each of the four non-officer directors of the Company stock options to purchase 10,000 shares (a total of 40,000 shares) of the Company’s common stock, exercisable for a period of five years at an exercise price of $5.88 per share (the closing market price on the grant date), vesting 12.5% on the last day of each subsequent calendar quarter-end until fully vested, subject to continued service. The fair value of these stock options, as calculated pursuant to the Black-Scholes option-pricing model, was determined to be $192,593 ($4.8131 per share), which is being charged to operations ratably from July 1, 2023 through June 30, 2025. For the year ended December 31, 2023, the Company recorded a total charge to general and administrative costs in the consolidated statement of operations of $48,464 with respect to these stock options.
On June 30, 2024, the Board of Directors, in accordance with the Company’s cash and equity compensation package for members of the Board of Directors, granted to each of the four non-officer directors of the Company stock options to purchase 10,000 shares (a total of 40,000 shares) of the Company’s common stock, exercisable for a period of five years at an exercise price of $2.37 per share (the closing market price on the grant date), vesting 12.5% on the last day of each subsequent calendar quarter-end until fully vested, subject to continued service. The fair value of these stock options, as calculated pursuant to the Black-Scholes option-pricing model, was determined to be $73,976 ($1.8494 per share), which is being charged to operations ratably from July 1, 2024 through June 30, 2026. During the year ended December 31, 2024, the Company record a charge general and administrative costs in the consolidated statement of operations of $18,648 with respect to these stock options.
On June 30, 2024, the Board of Directors, in conjunction with the Company’s efforts to preserve cash, granted to the four non-officer directors of the Company a total of 16,598 stock options to purchase shares of the Company’s common stock, exercisable for a period of five years at an exercise price of $2.37 per share (the closing market price on the grant date) The stock options were granted in lieu of cash compensation, are exercisable for a period of five years and were immediately vested. The number of stock options granted to each of the four non-officer directors of the Company was equal to the cash payment such director would otherwise have been entitled to receive for the quarter ended June 30, 2024, divided by their quarterly value as determined pursuant to the Black-Scholes option-pricing model, and was determined to be $27,500 ($1.6570 per share), which was charged to operations on June 30, 2024, the date on which the stock options were fully vested.
| -89- |
On September 30, 2024, the Board of Directors, in conjunction with the Company’s efforts to preserve cash, granted to the four non-officer directors of the Company a total of 21,217 stock options to purchase shares of the Company’s common stock, exercisable for a period of five years at an exercise price of $1.87 per share (the closing market price on the grant date) The stock options were granted in lieu of cash compensation, are exercisable for a period of five years and were immediately vested. The number of stock options granted to each of the four non-officer directors of the Company was equal to the cash payment such director would otherwise have been entitled to receive for the quarter ended September 30, 2024, divided by their quarterly value as determined pursuant to the Black-Scholes option-pricing model, and was determined to be $27,500 ($1.2961 per share), which was charged to operations on September 30, 2024, the date on which the stock options were fully vested.
On January 20, 2025, the Board of Directors, in conjunction with the Company’s efforts to preserve cash, granted to the four non-officer directors of the Company a total of 16,665 stock options to purchase shares of the Company’s common stock, exercisable for a period of five years at an exercise price of $2.33 per share (the closing market price on the grant date) The stock options were granted in lieu of cash compensation, are exercisable for a period of five years and were immediately vested. The number of stock options granted to each of the four non-officer directors of the Company was equal to the cash payment such director would otherwise have been entitled to receive for the quarter ended December 31, 2024, divided by their grant date value as determined pursuant to the Black-Scholes option-pricing model, and was determined to be $27,500 ($1.65002 per share). The grant date value of the stock options of $27,500 was accrued at December 31, 2024 and charged to operations at that date.
The table set forth below presents the compensation awarded to, earned by or paid to our named directors for the years ended December 31, 2024, 2023 and 2022.
DIRECTOR COMPENSATION TABLE
|
Name and Principal Position (2) |
Year | Salary ($) |
Bonus ($) |
Stock Awards ($) |
Option Awards ($)(1) |
Non-Equity Incentive Plan Compensation ($) |
Non-Qualified Deferred Compensation Earnings ($) |
All Other Compensation ($) |
Total ($) |
||||||||||||||||||||||||||
| Philip F. Palmedo | 2024 | - | - | - | - | - | - | - | - | ||||||||||||||||||||||||||
| Director (8) | 2023 | - | - | - | - | - | - | - | - | ||||||||||||||||||||||||||
| 2022 | - | - | - | 63,340 | - | - | 21,148 | 84,408 | |||||||||||||||||||||||||||
| Stephen J. Forman (9) | 2024 | - | - | - | 28,494 | - | - | 5,495 | 33,989 | ||||||||||||||||||||||||||
| Director | 2023 | - | - | - | 48,131 | - | - | 22,500 | 70,631 | ||||||||||||||||||||||||||
| 2022 | - | - | - | 63,340 | - | - | 22,500 | 85,840 | |||||||||||||||||||||||||||
| Yun Yen (3) | 2024 | - | - | - | 33,494 | - | - | 7,500 | 40,994 | ||||||||||||||||||||||||||
| Director | 2023 | - | - | - | 48,131 | - | - | 30,000 | 78,131 | ||||||||||||||||||||||||||
| 2022 | - | - | - | 63,340 | - | - | 30,000 | 93,340 | |||||||||||||||||||||||||||
| Gil Schwartzberg | 2024 | - | - | - | - | - | - | - | - | ||||||||||||||||||||||||||
| Director (4) | 2023 | - | - | - | - | - | - | - | - | ||||||||||||||||||||||||||
| 2022 | - | - | - | 63,340 | - | - | 16,630 | 79,970 | |||||||||||||||||||||||||||
| Regina Brown | 2024 | - | - | - | 34,744 | - | - | 7,630 | 42,374 | ||||||||||||||||||||||||||
| Director (5) | 2023 | - | - | - | 48,131 | - | - | 30,000 | 78,131 | ||||||||||||||||||||||||||
| 2022 | - | - | - | 63,340 | - | - | 30,000 | 93,340 | |||||||||||||||||||||||||||
| René Bernards | 2024 | - | - | - | 32,244 | - | - | 18,194 | 50,438 | ||||||||||||||||||||||||||
| Director (6) | 2023 | - | - | - | - | - | - | 62,500 | 62,500 | ||||||||||||||||||||||||||
| 2022 | - | - | - | - | - | - | 133,873 | 133,873 | |||||||||||||||||||||||||||
| Bas van der Baan | 2024 | - | - | - | - | - | - | - | - | ||||||||||||||||||||||||||
| Director (7) | 2023 | - | - | - | 48,131 | - | - | 18,478 | 66,609 | ||||||||||||||||||||||||||
| 2022 | - | - | - | 158,525 | - | - | 11,869 | 170,394 | |||||||||||||||||||||||||||
(1) Consists of grant date fair value of option award calculated pursuant to the Black-Scholes option-pricing model.
| -90- |
(2) Dr. John S. Kovach, the founder of the Company, served as Chairman of the Board of Directors until his death on October 5, 2023. Prior to September 26, 2023, Dr. Kovach was also the President, Chief Executive Officer and Chief Scientific Officer of the Company. Dr. Kovach did not receive any separate compensation for his services as a member of the Board of Directors.
(3) Appointed as a director of the Company effective August 4, 2018.
(4) Appointed as a director of the Company effective April 9, 2021 and died on October 30, 2022.
(5) Appointed as a director of the Company effective May 11, 2021.
(6) Appointed as a director of the Company effective June 15, 2022. Dr. Bernards received all of his compensation from June 15, 2022 through March 31, 2024 in the form of cash.
(7) Appointed as a director of the Company effective June 17, 2022, and as Chairman of the Board of Directors on October 6, 2023. Excludes compensation received after appointment as President and Chief Executive Officer on September 26, 2023.
(8) Did not stand for re-election at the annual meeting of stockholders. Accordingly, his term as a director of the Company ended effective October 7, 2022.
(9) Appointed as a director of the Company effective May 13, 2016.
Scientific Advisory Committee; Compensation
The Scientific Advisory Committee was established to advise the Company’s management in three areas: human molecular pathology; the clinical management of human brain tumors; and medicinal chemistry. Members of the Scientific Advisory Committee do not serve in any management capacity with the Company. During the years ended December 31, 2024, 2023 and 2022, the Scientific Advisory Committee consisted of one member, Dr. Daniel D. Von Hoff, M.D.
On December 24, 2013, the Company entered into a consulting agreement with NDA Consulting Corp. for consultation and advice in the field of oncology research and drug development. As part of the consulting agreement, NDA also agreed to have its president, Dr. Daniel D. Von Hoff, M.D., serve on the Company’s Scientific Advisory Committee during the term of such consulting agreement. The term of the consulting agreement was for one year and provided for a quarterly cash fee of $4,000. The consulting agreement had been automatically renewed for additional one-year terms on its anniversary date, most recently on December 24, 2023, but was subsequently terminated by mutual agreement effective September 30, 2024. As a result of the termination of the consulting agreement effective September 30, 2024, Dr. Von Hoff also ceased to be a member of the Scientific Advisory Committee at that time.
Consulting and advisory fees charged to operations pursuant to this consulting agreement were $12,000, $16,000 and $16,000 for the years ended December 31, 2024, 2023 and 2022, respectively, which were included in research and development costs in the consolidated statements of operations.
| -91- |
2020 Stock Incentive Plan
Summary
On July 14, 2020, the Board of Directors of the Company adopted the 2020 Stock Incentive Plan (the “2020 Plan”), which was subsequently approved by the stockholders of the Company. The 2020 Plan provides for the granting of equity-based awards, consisting of stock options, restricted stock, restricted stock units, stock appreciation rights, and other stock-based awards to employees, officers, directors and consultants of the Company and its affiliates, initially for a total of 233,333 shares of the Company’s common stock, under terms and conditions as determined by the Company’s Board of Directors. On October 7, 2022, the stockholders of the Company approved an amendment to the 2020 Plan to increase the number of common shares issuable thereunder by 180,000 shares, to a total of 413,333 shares. On November 27, 2023, the stockholders of the Company approved an amendment to the 2020 Plan to increase the number of common shares issuable thereunder by 336,667 shares, to a total of 750,000 shares.
As of December 31, 2024, unexpired stock options for 613,232 shares were issued and outstanding under the 2020 Plan and 136,768 shares were available for issuance under the 2020 Plan.
Having an adequate number of shares available for future equity compensation grants is necessary to promote our long-term success and the creation of stockholder value by:
| ● | Enabling us to continue to attract and retain the services of key service providers who would be eligible to receive grants; | |
| ● | Aligning the interests of participants with the interests of stockholders through incentives that are based upon the performance of our common stock; | |
| ● | Motivating participants, through equity incentive awards, to achieve long-term growth in our business, in addition to short-term financial performance; and | |
| ● | Providing a long-term equity incentive program that is competitive as compared to other companies with whom we compete for talent. |
The 2020 Plan permits the discretionary award of incentive stock options (“ISOs”), non-statutory stock options (“NQSOs”), restricted stock, restricted stock units (“RSUs”), stock appreciation rights (“SARs”), other equity awards and/or cash awards to selected participants. The 2020 Plan will remain in effect until July 14, 2030.
The 2020 Plan provides for the reservation of 750,000 shares of common stock for issuance thereunder (the “Share Limit”), and provides that the maximum number of shares that may be issued pursuant to the exercise of ISOs is 750,000 shares (the “ISO Limit”).
Key Features of the 2020 Plan
Certain key features of the 2020 Plan are summarized as follows:
| ● | If not terminated earlier by our Board of Directors, the 2020 Plan will terminate on July 14, 2030. | |
| ● | Up to a maximum aggregate of 750,000 shares of common stock may be issued under the 2020 Plan. The maximum number of shares that may be issued pursuant to the exercise of ISOs is also 750,000. | |
| ● | The 2020 Plan is administered by the Compensation Committee, which is comprised solely of independent members of our Board of Directors. The Board of Directors may designate a separate committee to make awards to employees who are not officers subject to the reporting requirements of Section 16 of the Exchange Act. |
| -92- |
| ● | Employees, consultants and board members are eligible to receive awards, provided that the Compensation Committee has the discretion to determine (i) who shall receive any awards, and (ii) the terms and conditions of such awards. | |
| ● | Awards may consist of ISOs, NQSOs, restricted stock, RSUs, SARs, other equity awards and/or cash awards. | |
| ● | Stock options and SARs may not be granted at a per share exercise price below the fair market value of a share of our common stock on the date of grant. | |
| ● | Stock options and SARs may not be repriced or exchanged without stockholder approval. | |
| ● | The maximum exercisable term of stock options and SARs may not exceed ten years. | |
| ● | Awards are subject to recoupment of compensation policies adopted by us. |
Eligibility to Receive Awards. Employees, consultants and members of our Board of Directors are eligible to receive awards under the 2020 Plan. The Compensation Committee determines, in its discretion, the selected participants who will be granted awards under the 2020 Plan.
Shares Subject to the 2020 Plan. The maximum number of shares of common stock that can be issued under the 2020 Plan is 750,000 shares.
The shares underlying forfeited or terminated awards (without payment of consideration), or unexercised awards become available again for issuance under the 2020 Plan. No fractional shares may be issued under the 2020 Plan. No shares will be issued with respect to a participant’s award unless applicable tax withholding obligations have been satisfied by the participant.
Administration of the 2020 Plan. The 2020 Plan is administered by the Compensation Committee of the Board of Directors, which consists of independent board members. With respect to certain awards issued under the 2020 Plan, the members of the Compensation Committee also must be “Non-Employee Directors” under Rule 16b-3 of the Exchange Act. Subject to the terms of the 2020 Plan, the Compensation Committee has the sole discretion, among other things, to:
| ● | Select the individuals who will receive awards; | |
| ● | Determine the terms and conditions of awards (for example, performance conditions, if any, and vesting schedule); |
| ● | Correct any defect, supply any omission, or reconcile any inconsistency in the 2020 Plan or any award agreement; | |
| ● | Accelerate the vesting, extend the post-termination exercise term or waive restrictions of any awards at any time and under such terms and conditions as it deems appropriate, subject to the limitations set forth in the 2020 Plan; | |
| ● | Permit a participant to defer compensation to be provided by an award; and | |
| ● | Interpret the provisions of the 2020 Plan and outstanding awards. |
The Compensation Committee may suspend vesting, settlement, or exercise of awards pending a determination of whether a selected participant’s service should be terminated for cause (in which case outstanding awards would be forfeited). Awards may be subject to any policy that the Board of Directors may implement on the recoupment of compensation (referred to as a “compensation clawback” policy). The members of the Board of Directors, the Compensation Committee and their delegates shall be indemnified by us to the maximum extent permitted by applicable law for actions taken or not taken regarding the 2020 Plan.
| -93- |
Types of Awards.
Stock Options. A stock option is the right to acquire shares at a fixed exercise price over a fixed period of time. The Compensation Committee determines, among other terms and conditions, the number of shares covered by each stock option and the exercise price of the shares subject to each stock option, but such per share exercise price cannot be less than the fair market value of a share of our common stock on the date of grant of the stock option. The exercise price of each stock option granted under the 2020 Plan must be paid in full at the time of exercise, either with cash, or through a broker-assisted “cashless” exercise and sale program, or net exercise, or through another method approved by the Compensation Committee. Stock options granted under the 2020 Plan may be either ISOs or NQSOs. In order to comply with Treasury Regulation Section 1.422-2(b), the 2020 Plan provides that no more than 750,000 shares may be issued pursuant to the exercise of ISOs.
SARs. A SAR is the right to receive, upon exercise, an amount equal to the difference between the fair market value of the shares on the date of the SAR’s exercise and the aggregate exercise price of the shares covered by the exercised portion of the SAR. The Compensation Committee determines the terms of SARs, including the exercise price (provided that such per share exercise price cannot be less than the fair market value of a share of our common stock on the date of grant), the vesting and the term of the SAR. Settlement of a SAR may be in shares of common stock or in cash, or any combination thereof, as the Compensation Committee may determine. SARs may not be repriced or exchanged without stockholder approval.
Restricted Stock. A restricted stock award is the grant of shares of our common stock to a selected participant and such shares may be subject to a substantial risk of forfeiture until specific conditions or goals are met. The restricted shares may be issued with or without cash consideration being paid by the selected participant as determined by the Compensation Committee. The Compensation Committee also will determine any other terms and conditions of an award of restricted stock.
RSUs. RSUs are the right to receive an amount equal to the fair market value of the shares covered by the RSU at some future date after the grant. The Compensation Committee will determine all of the terms and conditions of an award of RSUs. Payment for vested RSUs may be in shares of common stock or in cash, or any combination thereof, as the Compensation Committee may determine. RSUs represent an unfunded and unsecured obligation for us, and a holder of a stock unit has no rights other than those of a general creditor.
Other Awards. The 2020 Plan also provides that other equity awards, which derive their value from the value of our shares or from increases in the value of our shares, may be granted. In addition, cash awards may also be issued. Substitute awards may be issued under the 2020 Plan in assumption of or substitution for or exchange for awards previously granted by an entity which we may acquire.
Limited Transferability of Awards. Awards granted under the 2020 Plan generally are not transferrable other than by will or by the laws of descent and distribution. However, the Compensation Committee may in its discretion permit the transfer of awards other than ISOs.
Change in Control. In the event that we are a party to a merger or other reorganization or similar transaction, outstanding 2020 Plan awards will be subject to the agreement pertaining to such merger or reorganization. Such agreement may provide for (i) the continuation of the outstanding awards by us if we are a surviving corporation, (ii) the assumption or substitution of the outstanding awards by the surviving entity or its parent, (iii) full exercisability and/or full vesting of outstanding awards, or (iv) cancellation of outstanding awards either with or without consideration, in all cases with or without consent of the selected participant. The Compensation Committee will decide the effect of a change in control of us on outstanding awards.
Amendment and Termination of the 2020 Plan. The Board of Directors generally may amend or terminate the 2020 Plan at any time and for any reason, except that it must obtain stockholder approval of material amendments to the extent required by applicable laws, regulations or rules.
| -94- |
ITEM 12. SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT AND RELATED STOCKHOLDER MATTERS
The table set forth below presents certain information regarding beneficial ownership of our common stock (the only class of our voting equity securities issued and outstanding) as of March 14, 2025 by (i) each person or entity who is known by us to own beneficially more than 5% of our outstanding shares of common stock, (ii) each of our directors, and (iii) all of our directors and executive officers as a group. As of March 14, 2025, there were 2,684,074 shares of our common stock issued and outstanding. In computing the number and percentage of shares beneficially owned by a person, shares of common stock that a person has a right to acquire within sixty (60) days of March 14, 2025 pursuant to stock options, warrants, convertible preferred stock or other rights are counted as outstanding, while these shares are not counted as outstanding for computing the percentage ownership of any other person. This table is based upon information supplied by our directors, officers and principal stockholders and reports filed with the Securities and Exchange Commission. Except as noted, the Company’s executive office is reflected as the address of all officers, directors and other stockholders owning more than 5%.
| Name and Address of Beneficial Owner | Amount and Nature of Beneficial Ownership |
Percent of Class | ||||||
| Officers and Directors | ||||||||
| Bas van der Baan | ||||||||
| 680 East Colorado Boulevard, Suite 180 | ||||||||
| Pasadena, California 91101 | 168,498 | (2) | 5.9 | % | ||||
| Dr. Stephen J. Forman | ||||||||
| 680 East Colorado Boulevard, Suite 180 | ||||||||
| Pasadena, California 91101 | 52,711 | (3) | 1.9 | % | ||||
| Dr. Yun Yen | ||||||||
| 680 East Colorado Boulevard, Suite 180 | ||||||||
| Pasadena, California 91101 | 57,089 | (4) | 2.1 | % | ||||
| Dr. René Bernards | ||||||||
| 680 East Colorado Boulevard, Suite 180 | ||||||||
| Pasadena, California 91101 | 38,203 | (5) | 1.4 | % | ||||
| Regina Brown | ||||||||
| 680 East Colorado Boulevard, Suite 180 | ||||||||
| Pasadena, California 91101 | 68,053 | (10) | 2.5 | % | ||||
| Robert N. Weingarten | ||||||||
| 680 East Colorado Boulevard, Suite 180 | ||||||||
| Pasadena, California 91101 | 20,833 | (6) | 0.8 | % | ||||
| Dr. Jan H.M. Schellens | ||||||||
| 680 East Colorado Boulevard, Suite 180 | ||||||||
| Pasadena, California 91101 | 3,750 | (8) | 0.1 | % | ||||
| All officers and directors as a group (9 persons) | 409,137 | 13.4 | % | |||||
| Other Stockholders Owning More Than 5% | ||||||||
| John S. Kovach Trust | 156,128 | (1) | 5.8 | % | ||||
| 680 East Colorado Boulevard, Suite 180 | ||||||||
| Pasadena, California 91101 | ||||||||
| Barbara C. H. Kovach | 156,128 | (1) | 5.8 | % | ||||
| 680 East Colorado Boulevard, Suite 180 | ||||||||
| Pasadena, California 91101 | ||||||||
| Alexandra E. Kovach | 156,128 | (1) | 5.8 | % | ||||
| 680 East Colorado Boulevard, Suite 180 | ||||||||
| Pasadena, California 91101 | ||||||||
| Arthur and Jane Riggs 1990 Irrevocable Trust | ||||||||
| Jane Riggs, Trustee | ||||||||
| 4852 Saint Andres Avenue | ||||||||
| La Verne, California 91750 | 174,750 | (7) | 6.3 | % | ||||
| Glenn L. Krinsky | ||||||||
| 680 East Colorado Boulevard, Suite 180 | ||||||||
| Pasadena, California 91101 | 147,499 | (9) | 5.5 | % | ||||
(1) Includes 154,018 shares of common stock and stock warrants to purchase 2,110 shares of common stock owned by the John S. Kovach Trust dated September 22, 2015. The primary beneficiary of the trust is Barbara C. H. Kovach. Barbara C. H. Kovach and Alexandra E. Kovach are co-trustees of the trust and have the exclusive right to control the investment of the assets of the trust.
| -95- |
(2) Includes 11,000 shares of common stock and stock options to purchase 157,498 shares of common stock owned by Bas van der Baan.
(3) Includes 375 shares of common stock and stock options to purchase 43,126 shares of common stock owned by Dr. Stephen Forman. Also includes 7,105 shares of common stock and stock warrants to purchase 2,105 shares of common stock owned by the Stephen Forman Living Trust dated 12/16/98. Stephen Forman is trustee of the trust and holds voting and dispositive power over the common stock and common stock warrants owned by the trust.
(4) Includes 5,263 shares of common stock, stock warrants to purchase 5,263 shares of common stock and stock options to purchase 46,563 shares of common stock.
(5) Includes 25,000 shares of common stock and stock options to purchase 13,203 shares of common stock.
(6) Consists of stock options to purchase 20,833 shares of common stock.
(7) Includes 101,833 shares of common stock and 72,917 shares of common stock issuable upon conversion of 350,000 shares of Series A Convertible Preferred Stock owned by the Arthur and Jane Riggs 1990 Irrevocable Trust dated November 18, 1990. Jane Riggs is the trustee of the Arthur and Jane Riggs 1990 Irrevocable Trust. The shares of Series A Convertible Preferred Stock were acquired on March 17, 2015 and January 15, 2016, are non-voting, and are immediately convertible into common stock.
(8) Consists of stock options to purchase 3,750 shares of common stock.
(9) Includes 14,166 shares of common stock owned by Glenn L. Krinsky. Also includes 133,333 shares of common stock owned by the John and Barbara Kovach 2015 Trust, as to which Glenn L. Krinsky, as trustee, has voting, dispositive and investment control.
(10) Includes 630 shares of common stock and stock options to purchase 67,423 shares of common stock.
ITEM 13. CERTAIN RELATIONSHIPS AND RELATED PARTY TRANSACTIONS, AND DIRECTOR INDEPENDENCE
(a) Related Party Transactions
During the years ended December 31, 2024, 2023,and 2022, there were no transactions, either directly or indirectly, between the Company and any of its officers, directors or affiliates, including their family members, except as described elsewhere in this document.
| -96- |
(b) Director Independence
The Company considers that Dr. Yun Yen, Regina Brown and Dr. René Bernards are each an “independent director,” as defined under Nasdaq rules and by Rule 10A-3 of the Exchange Act.
ITEM 14. PRINCIPAL ACCOUNTANT FEES AND SERVICES
Weinberg & Company, P.A. acted as the Company’s independent registered public accounting firm for the fiscal years ended December 31, 2024 and 2023 and for the interim periods in such fiscal years. The following table shows the fees that were incurred by the Company for audit and other services provided by Weinberg & Company, P.A. for the years ended December 31, 2024 and 2023.
| Years Ended December 31, | ||||||||
| 2024 | 2023 | |||||||
| Audit Fees(1) | $ | 104,205 | $ | 120,640 | ||||
| Audit-Related Fees(2) | — | — | ||||||
| Tax Fees(3) | 13,718 | 32,860 | ||||||
| Other Fees(4) | 25,695 | — | ||||||
| Total | $ | 143,618 | $ | 153,500 | ||||
| (1) | Audit fees represent fees for professional services provided in connection with the audit of the Company’s annual financial statements included in its Annual Reports on Form 10-K and the review of its interim financial statements included in its Quarterly Reports on Form 10-Q and services that are normally provided in connection with statutory or regulatory filings, excluding those fees included in Other Fees. |
| (2) | Audit-related fees represent fees for assurance and related services that are reasonably related to the performance of the audit or review of the Company’s financial statements and not reported above under Audit Fees. |
| (3) | Tax fees represent fees for professional services related to tax compliance, tax advice and tax planning. |
| (4) | Other fees represent fees incurred with respect to the Company’s Registration Statements on Form S-1 and Form S-3. |
All audit and audit-related services, tax services and other services rendered by Weinberg & Company, P.A. during the fiscal years ended December 31, 2024 and 2023 were pre-approved by either the Company’s Audit Committee or by the Company’s Board of Directors. The Board of Directors has adopted a pre-approval policy that provides for the pre-approval of all services performed for the Company by its independent registered public accounting firm.
| -97- |
PART IV
ITEM 15. EXHIBITS AND FINANCIAL STATEMENT SCHEDULES
| (a) | List of documents filed as part of this report: |
| (1) | Financial Statements |
Reference is made to the Index to Consolidated Financial Statements on page F-1, where these documents are listed.
| (2) | Financial Statement Schedules |
The financial statement schedules have been omitted because the required information is not applicable, or not present in amounts sufficient to require submission of the schedules, or because the information is included in the financial statements or notes thereto.
| (3) | Exhibits |
See (b) below.
| (b) | Exhibits: |
A list of exhibits required to be filed as part of this Annual Report on Form 10-K is set forth in the Index to Exhibits, which is presented elsewhere in this document, and is incorporated herein by reference.
ITEM 16. FORM 10-K SUMMARY
None
| -98- |
INDEX TO EXHIBITS
| -99- |
| -100- |
| -101- |
| * | Filed herewith. |
| + | Indicates a management contract or any compensatory plan, contract or arrangement. |
| -102- |
SIGNATURES
In accordance with Section 13 and 15(d) of the Securities Exchange Act of 1934, the Registrant caused this report to be signed on its behalf by the undersigned thereunto duly authorized.
| Date: March 24, 2025 | LIXTE BIOTECHNOLOGY HOLDINGS, INC. | |
| (Registrant) | ||
| By: | /s/ BASTIAAN VAN DER BAAN | |
| Name: | Bastiaan van der Baan | |
| Title: | President and Chief Executive Officer |
In accordance with the Securities Exchange Act of 1934, this Report has been signed below by the following persons on behalf of the Registrant in the capacity and on the dates indicated.
| Signature | Title | Date | ||
| /s/ BASTIAAN VAN DER BAAN | President and Chief Executive Officer | March 24, 2025 | ||
|
John S. Kovach |
||||
| /s/ ROBERT N. WEINGARTEN | Vice President and Chief Financial Officer | March 24, 2025 | ||
|
Robert N. Weingarten
|
||||
| /s/ STEPHEN J. FORMAN | Director | March 24, 2025 | ||
| Stephen J. Forman | ||||
| /s/ RENE BERNARDS | Director | March 24, 2025 | ||
| René Bernards | ||||
| /s/ YUN YEN | Director | March 24, 2025 | ||
| Yun Yen | ||||
| /s/ REGINA BROWN | Director | March 24, 2025 | ||
| Regina Brown |
| -103- |
LIXTE BIOTECHNOLOGY HOLDINGS, INC.
AND SUBSIDIARY
INDEX TO CONSOLIDATED FINANCIAL STATEMENTS
(INCLUDING REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM)
Years Ended December 31, 2024 and 2023
| F-1 |
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
To the Stockholders and Board of Directors
Lixte Biotechnology Holdings, Inc.
Opinion on the Financial Statements
Going Concern
The accompanying financial statements have been prepared assuming that the Company will continue as a going concern. As discussed in Note 1 to the financial statements, the Company has no recurring source of revenue and has experienced negative operating cash flows since inception. The Company has financed its working capital requirements through the recurring sale of its equity securities. These matters raise substantial doubt about the Company’s ability to continue as a going concern. Management’s plans in regard to these matters are also described in Note 1 to the financial statements. These financial statements do not include any adjustments that might result from the outcome of this uncertainty.
Basis for Opinion
These financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on the Company’s financial statements based on our audits. We are a public accounting firm registered with the Public Company Accounting Oversight Board (United States) (“PCAOB”) and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audits in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement, whether due to error or fraud. The Company is not required to have, nor were we engaged to perform, an audit of its internal control over financial reporting. As part of our audits we are required to obtain an understanding of internal control over financial reporting but not for the purpose of expressing an opinion on the effectiveness of the Company’s internal control over financial reporting. Accordingly, we express no such opinion.
Our audits included performing procedures to assess the risks of material misstatement of the financial statements, whether due to error or fraud, and performing procedures that respond to those risks. Such procedures included examining, on a test basis, evidence regarding the amounts and disclosures in the financial statements. Our audits also included evaluating the accounting principles used and significant estimates made by management, as well as evaluating the overall presentation of the financial statements. We believe that our audits provide a reasonable basis for our opinion.
Critical Audit Matter
The critical audit matter communicated below is a matter arising from the current period audit of the financial statements that was communicated or required to be communicated to the audit committee and that (1) relates to accounts or disclosures that are material to the financial statements and (2) involved our especially challenging, subjective, or complex judgments. The communication of the critical audit matter does not alter in any way our opinion on the financial statements, taken as a whole, and we are not, by communicating the critical audit matter below, providing a separate opinion on the critical audit matters or on the accounts or disclosures to which it relates.
| F-2 |
Stock-Based Compensation
As discussed in Note 6 to the financial statements, the Company recognized $418,422 of compensation expense related to stock-based awards to certain officers, employees and consultants. Management accounts for stock-based compensation based on the estimated fair value of each award granted, which is amortized as expense over the requisite service period of the award.
Auditing management’s estimate of the valuation of stock-based compensation was complex and highly judgmental due to the subjectivity of the inputs and assumptions that management utilized in determining the fair value of the stock-based awards.
Our audit procedures related to the stock-based awards, including the valuation methodology and related assumptions such as the risk-free interest rate, volatility, and dividend yield, consisted of the following, among others:
| ● | We obtained and read the stock-based award agreements, and obtained board minutes and board resolutions related to the stock-based awards. | |
| ● | We evaluated the option price model management selected to determine the fair value, and evaluated the reasonableness of management’s significant valuation assumptions, and tested the mathematical accuracy of management’s valuation analyses. | |
| ● | We developed independent estimates for the fair values of the stock-based awards. |
We have served as the Company’s auditor since 2008.
/s/
March 24, 2025
| F-3 |
LIXTE BIOTECHNOLOGY HOLDINGS, INC.
AND SUBSIDIARY
CONSOLIDATED BALANCE SHEETS
| December 31, | ||||||||
| 2024 | 2023 | |||||||
| ASSETS | ||||||||
| Current assets: | ||||||||
| Cash | $ | $ | ||||||
| Advances on research and development contract services | ||||||||
| Prepaid insurance | ||||||||
| Other prepaid expenses | ||||||||
| Total assets | $ | $ | ||||||
| LIABILITIES AND STOCKHOLDERS’ EQUITY | ||||||||
| Current liabilities: | ||||||||
| Accounts payable and accrued expenses, including $ |
$ | $ | ||||||
| Research and development contract liabilities, including $ |
||||||||
| Total current liabilities | ||||||||
| Commitments and contingencies | ||||||||
| Stockholders’ equity: | ||||||||
| Preferred Stock, $ par value; authorized – shares; issued and outstanding – shares of Series A Convertible Preferred Stock, $ |
||||||||
| Common stock, $ par value; authorized – shares; issued and outstanding – shares at December 31, 2024 and 2023 | ||||||||
| Additional paid-in capital | ||||||||
| Accumulated deficit | ( |
) | ( |
) | ||||
| Total stockholders’ equity | ||||||||
| Total liabilities and stockholders’ equity | $ | $ | ||||||
See accompanying notes to consolidated financial statements.
| F-4 |
LIXTE BIOTECHNOLOGY HOLDINGS, INC.
AND SUBSIDIARY
CONSOLIDATED STATEMENTS OF OPERATIONS
| Years Ended December 31, | ||||||||
| 2024 | 2023 | |||||||
| Revenues | $ | $ | ||||||
| Costs and expenses: | ||||||||
| Research and development costs | ||||||||
| General and administrative costs | ||||||||
| Total costs and expenses | ||||||||
| Loss from operations | ( |
) | ( |
) | ||||
| Interest income | ||||||||
| Interest expense | ( |
) | ( |
) | ||||
| Foreign currency gain (loss) | ( |
) | ||||||
| Net loss | $ | ( |
) | $ | ( |
) | ||
| Net loss per common share – basic and diluted | $ | ) | $ | ) | ||||
| Weighted average common shares outstanding – basic and diluted | ||||||||
See accompanying notes to consolidated financial statements.
| F-5 |
LIXTE BIOTECHNOLOGY HOLDINGS, INC.
AND SUBSIDIARY
CONSOLIDATED STATEMENTS OF STOCKHOLDERS’ EQUITY
Years Ended December 31, 2024 and 2023
|
Series A Convertible Preferred Stock |
Common Stock | Additional Paid-in | Accumulated | Total Stockholders’ | ||||||||||||||||||||||||
| Shares | Amount | Shares | Par Value | Capital | Deficit | Equity | ||||||||||||||||||||||
| Balance, December 31, 2022 | $ | $ | $ | $ | ( |
) | $ | |||||||||||||||||||||
| Proceeds from sale of securities in registered direct equity offering, net of offering costs | — | |||||||||||||||||||||||||||
| Exercise of pre-funded common stock warrants | — | |||||||||||||||||||||||||||
| Exercise of common stock options | — | |||||||||||||||||||||||||||
| Stock-based compensation | — | — | ||||||||||||||||||||||||||
| Net loss | — | — | ( |
) | ( |
) | ||||||||||||||||||||||
| Balance, December 31, 2023 | ( |
) | ||||||||||||||||||||||||||
| Stock-based compensation | — | — | ||||||||||||||||||||||||||
| Net loss | — | — | ( |
) | ( |
) | ||||||||||||||||||||||
| Balance, December 31, 2024 | $ | $ | $ | $ | ( |
) | $ | |||||||||||||||||||||
See accompanying notes to consolidated financial statements.
| F-6 |
LIXTE BIOTECHNOLOGY HOLDINGS, INC.
AND SUBSIDIARY
CONSOLIDATED STATEMENTS OF CASH FLOWS
| Years Ended December 31, | ||||||||
| 2024 | 2023 | |||||||
| Cash flows from operating activities: | ||||||||
| Net loss | $ | ( |
) | $ | ( |
) | ||
| Adjustments to reconcile net loss to net cash used in operating activities: | ||||||||
| Stock-based compensation expense included in - | ||||||||
| General and administrative costs | ||||||||
| Research and development costs | ||||||||
| Changes in operating assets and liabilities: | ||||||||
| (Increase) decrease in - | ||||||||
| Advances on research and development contract services | ||||||||
| Prepaid insurance | ( |
) | ||||||
| Other prepaid expenses | ( |
) | ||||||
| Increase (decrease) in - | ||||||||
| Accounts payable and accrued expenses | ( |
) | ( |
) | ||||
| Research and development contract liabilities | ( |
) | ||||||
| Net cash used in operating activities | ( |
) | ( |
) | ||||
| Cash flows from financing activities: | ||||||||
| Proceeds from sale of securities in registered direct offering, net of offering costs |
||||||||
| Exercise of pre-funded common stock warrants | ||||||||
| Exercise of common stock options | ||||||||
| Net cash provided by financing activities | ||||||||
| Cash: | ||||||||
| Net decrease | ( |
) | ( |
) | ||||
| Balance at beginning of period | ||||||||
| Balance at end of period | $ | $ | ||||||
| Supplemental disclosures of cash flow information: | ||||||||
| Cash paid for - | ||||||||
| Interest | $ | $ | ||||||
| Income taxes | $ | $ | ||||||
See accompanying notes to consolidated financial statements.
| F-7 |
LIXTE BIOTECHNOLOGY HOLDINGS, INC.
AND SUBSIDIARY
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
Years Ended December 31, 2024 and 2023
1. Organization and Basis of Presentation
Lixte Biotechnology Holdings, Inc., a Delaware corporation, including its wholly-owned Delaware subsidiary, Lixte Biotechnology, Inc. (collectively, the “Company”), is a clinical-stage biopharmaceutical company focused on identifying new targets for cancer drug development and developing and commercializing cancer therapies. The Company’s corporate office is located in Pasadena, California.
The Company’s product pipeline is primarily focused on inhibitors of protein phosphatase 2A, which is used to enhance cytotoxic agents, radiation, immune checkpoint blockers and other cancer therapies. The Company believes that inhibitors of protein phosphatases have significant therapeutic potential for a broad range of cancers. The Company is focusing on the clinical development of a specific protein phosphatase inhibitor, referred to as LB-100, which has been shown to have clinical anti-cancer activity.
The Company’s activities are subject to significant risks and uncertainties, including the need for additional capital. The Company has not yet commenced any revenue-generating operations, does not have positive cash flows from operations, relies on stock-based compensation for a substantial portion of employee and consultant compensation, and is dependent on periodic access to equity capital to fund its operating requirements.
Reverse Stock Split
On June 2, 2023, the Company effected a
Nasdaq Compliance
The Company’s common stock and the warrants are traded on the Nasdaq Capital Market under the symbols “LIXT” and “LIXTW”, respectively.
On
June 2, 2023, the Company effected a
On
August 23, 2024, the Company received a letter from the Listing Qualifications Department (the “Staff”) of the Nasdaq Stock
Market LLC (“Nasdaq”) on August 19, 2024 indicating that the Company was not in compliance with the minimum stockholders’
equity requirement of $
On October 3, 2024, the Company submitted a plan to the Staff to regain compliance with the Stockholders’ Equity Requirement, which outlined the Company’s proposed initiatives to regain compliance by raising equity capital through various registered equity offerings.
On October 21, 2024, the Staff provided notice (the “Notice”) to the Company that it had granted an extension through February 18, 2025 to regain compliance with the Stockholders’ Equity Requirement, which required that the Company complete its capital raising initiatives and evidence compliance with the Stockholders’ Equity Requirement through filing a Current Report on Form 8-K with the Securities and Exchange Commission (the “SEC”) providing certain required information.
As of February 18, 2025, the Company had not gained compliance with the Stockholders’ Equity Requirement. Accordingly, on February 19, 2025, the Company received a Staff determination letter from the Staff stating that the Company did not meet the terms of the extension because it did not complete its proposed financing initiatives to regain compliance.
| F-8 |
The Company timely filed an appeal and requested a Hearing before a Nasdaq Hearings Panel (the “Panel”), which has been granted. The Hearing request automatically stayed Nasdaq’s delisting of the Company’s common shares and warrants pending the Panel’s decision. Pursuant to the Nasdaq Listing Rules, the Panel has the discretion to grant the Company an additional extension through no later than August 18, 2025. At the upcoming hearing, the Company will present its plan for regaining and sustaining compliance with the Stockholders’ Equity Requirement for continued listing. However, there can be no assurances that the Hearings Panel will grant the Company an extension of time to regain compliance, or that the Company will be able to regain compliance during any extension period. During the appeal process the Company’s common shares and warrants will continue to trade on The Nasdaq Capital Market.
The Company intends to take reasonable measures available to regain compliance under Nasdaq’s listing rules and to remain listed on Nasdaq. However, there can be no assurances that the Company will ultimately regain compliance with the Stockholders’ Equity Rule, or be able to maintain compliance with all other applicable requirements for continued listing on Nasdaq. If the Company does not regain compliance with Nasdaq’s continued listing requirements within the time period permitted by Nasdaq, then the Company’s securities will be delisted from Nasdaq.
Going Concern
For
the year ended December 31, 2024, the Company recorded a net loss of $
Because
the Company is currently engaged in various early-stage clinical trials, it is expected that it will take a significant amount of time
and resources to develop any product or intellectual property capable of generating sustainable revenues. Accordingly, the Company’s
business is unlikely to generate any sustainable operating revenues in the next several years and may never do so. Even if the Company
is able to generate revenues through licensing its technology, product sales or other commercial activities, there can be no assurance
that the Company will be able to achieve and maintain positive earnings and operating cash flows. At March 14, 2025, the Company’s
remaining financial contractual commitments pursuant to clinical trial agreements and clinical trial monitoring agreements not yet incurred
aggregated approximately $
The Company’s consolidated financial statements have been presented on the basis that it will continue as a going concern, which contemplates the realization of assets and satisfaction of liabilities in the normal course of business. The consolidated financial statements also do not reflect any adjustments relating to the recoverability of assets and liabilities that might be necessary if the Company is unable to continue as a going concern. The Company has no recurring source of revenues and has experienced negative operating cash flows since inception. The Company has financed its working capital requirements through the recurring sale of its equity securities.
| F-9 |
Based on the foregoing, management has concluded that there is substantial doubt about the Company’s ability to continue as a going concern within one year after the date that the consolidated financial statements are being issued. In addition, our independent registered public accounting firm has included an explanatory paragraph in their report with respect to this uncertainty that accompanies our audited consolidated financial statements as of and for the year ended December 31, 2024. The Company’s consolidated financial statements do not include any adjustments that might result from the outcome of this uncertainty.
The Company’s ability to continue as a going concern is dependent upon its ability to raise additional equity capital to fund its research and development activities and to ultimately achieve sustainable operating revenues and profitability. The amount and timing of future cash requirements depends on the pace, design and results of the Company’s clinical trial program, which, in turn, depends on the availability of operating capital to fund such activities.
Based on current operating plans, the Company estimates that its existing cash resources at December 31, 2024, and the funds raised subsequent to December 31, 2024, will provide sufficient working capital to fund the current clinical trial program with respect to the development of the Company’s lead anti-cancer clinical compound LB-100 through approximately September 30, 2025. However, existing cash resources will not be sufficient to complete the development of and obtain regulatory approval for the Company’s product candidate, which will require that the Company raise significant additional capital. The Company estimates that it will need to raise additional capital to fund its operations by mid-2025 to be able to proactively manage its current business plan during the remainder of 2025 and during 2026. In addition, the Company’s operating plans may change as a result of many factors that are currently unknown and/or outside of the control of the Company, and additional funds may be needed sooner than planned. The Company is considering various strategies and alternatives to obtain the required additional capital. However, as market conditions present uncertainty as to the Company’s ability to secure additional funds, there can be no assurance that the Company will be able to secure additional financing on acceptable terms, as and when necessary, to continue to conduct operations.
If cash resources are insufficient to satisfy the Company’s ongoing cash requirements, the Company would be required to scale back or discontinue its clinical trial program, as well as its licensing and patent prosecution efforts and its technology and product development efforts, or obtain funds, if available, through strategic alliances, joint ventures or other transaction structures that could require the Company to relinquish rights to and/or control of LB-100, or to curtail or discontinue operations entirely.
2. Summary of Significant Accounting Policies
Principles of Consolidation
The accompanying consolidated financial statements of the Company have been prepared in accordance with United States generally accepted accounting principles (“GAAP”) and include the financial statements of Lixte Biotechnology Holdings, Inc. and its wholly-owned subsidiary, Lixte Biotechnology, Inc. Intercompany balances and transactions have been eliminated in consolidation.
Segment Information
The Company’s President and Chief Executive Officer is the Company’s Chief Operating Decision Maker (“CODM”) and evaluates performance and makes operating decisions about allocating resources based on internal financial data presented on a consolidated basis. Because the CODM evaluates financial performance on a consolidated basis, the Company has determined that it operates in a single reportable segment, which consists of the development of a drug class called Protein Phosphatase 2A inhibitors, and is comprised of the consolidated financial results of the Company. The CODM uses consolidated net income (loss) as the sole measure of segment profit or loss. The required segment information, including significant segment expenses, is presented at Note 3.
Use of Estimates
The preparation of financial statements in conformity with GAAP requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities at the date of the financial statements and the reported amounts of expenses during the reporting period. Some of those judgments can be subjective and complex, and therefore, actual results could differ materially from those estimates under different assumptions or conditions. Management bases its estimates on historical experience and on various assumptions that are believed to be reasonable in relation to the financial statements taken, as a whole, under the circumstances, the results of which form the basis for making judgments about the carrying values of assets and liabilities that are not readily apparent from other sources. Management regularly evaluates the key factors and assumptions used to develop the estimates utilizing currently available information, changes in facts and circumstances, historical experience, and reasonable assumptions. After such evaluations, if deemed appropriate, those estimates are adjusted accordingly. Actual results could differ from those estimates. Significant estimates include those related to assumptions used in the calculation of accruals for clinical trial costs and other potential liabilities, and valuing equity instruments issued for services.
| F-10 |
Cash
Cash
is held in a cash bank deposit program maintained by Morgan Stanley Wealth Management, a division of Morgan Stanley Smith Barney LLC
(“Morgan Stanley”). Morgan Stanley is a FINRA-regulated broker-dealer. The Company’s policy is to maintain its cash
balances with financial institutions in the United States with high credit ratings and in accounts insured by the Federal Deposit Insurance
Corporation (the “FDIC”) and/or by the Securities Investor Protection Corporation (the “SIPC”). The Company periodically
has cash balances in financial institutions in excess of the FDIC and SIPC insurance limits of $
Research and Development
Research and development costs consist primarily of fees paid to consultants and contractors, and other expenses relating to the negotiation, design, development, conduct and management of clinical trials with respect to the Company’s clinical compound and product candidate. Research and development costs also include the costs to manufacture compounds used in research and clinical trials, which are charged to operations as incurred. The Company’s inventory of LB-100 for clinical use has been manufactured separately in the United States and in the European Union in accordance with the laws and regulations of such jurisdictions.
Research and development costs are generally charged to operations ratably over the life of the underlying contracts, unless the achievement of milestones, the completion of contracted work, the termination of an agreement, or other information indicates that a different expensing schedule is more appropriate. However, payments for research and development costs that are contractually defined as non-refundable are charged to operations as incurred.
Obligations incurred with respect to mandatory scheduled payments under agreements with milestone provisions are recognized as charges to research and development costs in the Company’s consolidated statement of operations based on the achievement of such milestones, as specified in the respective agreement. Obligations incurred with respect to mandatory scheduled payments under agreements without milestone provisions are accounted for when due, are recognized ratably over the appropriate period, as specified in the respective agreement, and are recorded as liabilities in the Company’s consolidated balance sheet, with a corresponding charge to research and development costs in the Company’s consolidated statement of operations.
Payments made pursuant to contracts are initially recorded as advances on research and development contract services in the Company’s consolidated balance sheet and are then charged to research and development costs in the Company’s consolidated statement of operations as those contract services are performed. Expenses incurred under contracts in excess of amounts advanced are recorded as research and development contract liabilities in the Company’s consolidated balance sheet, with a corresponding charge to research and development costs in the Company’s consolidated statement of operations. The Company reviews the status of its various clinical trial and research and development contracts on a quarterly basis.
Prepaid Insurance
Prepaid insurance represents the premiums paid for directors and officers insurance coverage and for general liability insurance coverage in excess of the amortization of the total policy premium charged to operations at each balance sheet date. Such amount is determined by amortizing the total policy premium charged on a straight-line basis over the respective policy period. As the policy premiums incurred are generally amortizable over the ensuing twelve-month period, they are recorded as a current asset in the Company’s consolidated balance sheet at each reporting date and appropriately amortized to the Company’s consolidated statement of operations for each reporting period.
Offering Costs
Offering costs consist of costs incurred with respect to equity financing transactions, including legal fees. Such costs are deferred and charged to additional paid-in capital upon the successful completion of such financings, or are charged to operations if and when such financings are abandoned or terminated.
| F-11 |
Patent and Licensing Legal and Filing Fees and Costs
Due
to the significant uncertainty associated with the successful development of commercially viable products based on the Company’s
research efforts and related patent applications, all patent and licensing legal and filing fees and costs related to the development
and protection of the Company’s intellectual property are charged to operations as incurred. Patent and licensing legal and filing
fees and costs were $
Concentration of Risk
The
Company periodically contracts with vendors and consultants to provide services related to the Company’s operations. Charges incurred
for these services can be for a specific period (typically one year) or for a specific project or task. Costs and expenses incurred that
represented
General
and administrative costs for the years ended December 31, 2024 and 2023 include charges from legal firms and other vendors for general
licensing and patent prosecution costs relating to the Company’s intellectual properties representing
Research
and development costs for the year ended December 31, 2024 include charges from three vendors and consultants representing
Income Taxes
The Company accounts for income taxes under an asset and liability approach for financial accounting and reporting for income taxes. Accordingly, the Company recognizes deferred tax assets and liabilities for the expected impact of differences between the financial statements and the tax basis of assets and liabilities.
The Company records a valuation allowance to reduce its deferred tax assets to the amount that is more likely than not to be realized. Due to the uncertainty of the Company’s ability to realize the benefit of the deferred tax assets, the net deferred tax assets are fully offset by a valuation allowance at December 31, 2024 and 2023. In the event the Company was to determine that it would be able to realize its deferred tax assets in the future in excess of its recorded amount, an adjustment to the deferred tax assets would be credited to operations in the period such determination was made. Should the Company determine that it would not be able to realize all or part of its deferred tax assets in the future, an adjustment to the deferred tax assets would be charged to operations in the period such determination was made.
The Company is subject to U.S. federal income taxes and income taxes of various state tax jurisdictions. As the Company’s net operating losses have yet to be utilized, all previous tax years remain open to examination by Federal authorities and other jurisdictions in which the Company currently operates or has operated in the past. The Company had no unrecognized tax benefits as of December 31, 2024 or 2023 and does not anticipate any material amount of unrecognized tax benefits through December 31, 2025.
The Company accounts for uncertainties in income tax law under a comprehensive model for the financial statement recognition, measurement, presentation, and disclosure of uncertain tax positions taken or expected to be taken in income tax returns as prescribed by GAAP. The tax effects of a position are recognized only if it is “more-likely-than-not” to be sustained by the taxing authority as of the reporting date. If the tax position is not considered “more-likely-than-not” to be sustained, then no benefits of the position are recognized. The Company had not recorded any liability for uncertain tax positions as of December 31, 2024 or 2023. Subsequent to December 31, 2024, any interest and penalties related to uncertain tax positions will be recognized as a component of income tax expense.
| F-12 |
The Company periodically issues common stock and stock options to officers, directors, employees, contractors and consultants for services rendered. Options vest and expire according to terms established at the issuance date of each grant. Stock grants, which are generally time vested, are measured at the grant date fair value and charged to operations ratably over the vesting period.
The Company accounts for stock-based payments to officers, directors, employees, contractors, and consultants by measuring the cost of services received in exchange for equity awards utilizing the grant date fair value of the awards, with the cost recognized as compensation expense on the straight-line basis in the Company’s financial statements over the vesting period of the awards. Recognition of compensation expense for non-employees is in the same period and manner as if the Company had paid cash for the services.
The fair value of stock options granted as stock-based compensation is determined utilizing the Black-Scholes option-pricing model, and is affected by several variables, the most significant of which are the expected life of the stock option, the exercise price of the stock option as compared to the fair market value of the common stock on the grant date, and the estimated volatility of the common stock. Unless sufficient historical exercise data is available, the expected life of the stock option is calculated as the mid-point between the vesting period and the contractual term (the “simplified method”). The estimated volatility is based on the historical volatility of the Company’s common stock, calculated utilizing a look-back period approximately equal to the contractual life of the stock option being granted. The risk-free interest rate is based on the U.S. Treasury yield curve in effect at the time of grant. The fair market value of the common stock is determined by reference to the quoted market price of the Company’s common stock on the grant date. The expected dividend yield is based on the Company’s expectation of dividend payouts and is assumed to be zero.
The Company recognizes the fair value of stock-based compensation awards in general and administrative costs and in research and development costs, as appropriate, in the Company’s consolidated statements of operations. The Company issues new shares of common stock to satisfy stock option exercises.
Warrants
The Company accounts for warrants as either equity-classified or liability-classified instruments based on an assessment of the warrant’s specific terms and applicable authoritative guidance in Accounting Standards Codification (“ASC”) 480, Distinguishing Liabilities from Equity (“ASC 480”), and ASC 815, Derivatives and Hedging (“ASC 815”). The assessment considers whether the warrants are freestanding financial instruments pursuant to ASC 480, meet the definition of a liability pursuant to ASC 480, and whether the warrants meet all of the requirements for equity classification under ASC 815, including whether the warrants are indexed to the Company’s own common stock and whether the warrant holders could potentially require “net cash settlement” in a circumstance outside of the Company’s control, among other conditions for equity classification. The Company has determined that the warrants issued in the July 20, 2023 equity financing (see Note 4) meet the requirements for equity classification. This assessment, which requires the use of professional judgment, is conducted when the warrants are issued and at the end each subsequent quarterly period while the warrants are outstanding. For issued or modified warrants that meet all of the criteria for equity classification, the warrants are required to be recorded as a component of additional paid-in capital at the time of issuance. For issued or modified warrants that do not meet all of the criteria for equity classification, the warrants are required to be liability-classified and recorded at their initial fair value on the date of issuance and remeasured at fair value at each balance sheet date thereafter. Changes in the estimated fair value of the warrants that are liability-classified are recognized as a non-cash gain or loss in the statement of operations at each balance sheet date. At December 31, 2024 and 2023, the Company did not have any liability-classified warrants.
The Company’s computation of earnings (loss) per share (“EPS”) includes basic and diluted EPS. Basic EPS is measured as the income (loss) attributable to common stockholders divided by the weighted average common shares outstanding for the period. Diluted EPS is similar to basic EPS but presents the dilutive effect on a per share basis of potential common shares (e.g., preferred shares, warrants and stock options) as if they had been converted at the beginning of the respective periods presented, or issuance date, if later. Potential common shares that have an anti-dilutive effect (i.e., those that increase income per share or decrease loss per share) are excluded from the calculation of diluted EPS.
| F-13 |
Loss per common share is computed by dividing net loss by the weighted average number of common shares outstanding during the respective periods. Basic and diluted loss per common share was the same for all periods presented because all preferred shares, warrants and stock options outstanding were anti-dilutive.
| December 31, | ||||||||
| 2024 | 2023 | |||||||
| Series A Convertible Preferred Stock | ||||||||
| Common stock warrants | ||||||||
| Common stock options, including options issued in the form of warrants | ||||||||
| Total | ||||||||
Foreign Currency Translation
The consolidated financial statements are presented in the United States dollar, which is the functional and reporting currency of the Company.
The Company periodically incurs a cost or expense in a foreign jurisdiction denominated in a local currency. The Company purchases the required foreign currency to pay such cost or expense on an as-needed basis. Such cost or expense is converted into United States dollars for financial statement purposes based on the foreign currency conversion rate in effect on the transaction date. The Company purchases the requisite foreign currency to pay such cost or expense on an as-needed basis. Any gain or loss resulting from the purchase of the foreign currency is included as foreign currency gain (loss) in the consolidated statement of operations.
During
the years ended December 31, 2024 and 2023, the Company incurred various costs and expenses denominated in Euros, which were converted
into United States dollars at the average rate of
Fair Value of Financial Instruments
The authoritative guidance with respect to fair value established a fair value hierarchy that prioritizes the inputs to valuation techniques used to measure fair value into three levels and requires that assets and liabilities carried at fair value be classified and disclosed in one of three categories, as presented below. Disclosure as to transfers in and out of Levels 1 and 2, and activity in Level 3 fair value measurements, is also required.
Level 1. Observable inputs such as quoted prices in active markets for an identical asset or liability that the Company has the ability to access as of the measurement date. Financial assets and liabilities utilizing Level 1 inputs include active-exchange traded securities and exchange-based derivatives.
Level 2. Inputs, other than quoted prices included within Level 1, which are directly observable for the asset or liability or indirectly observable through corroboration with observable market data. Financial assets and liabilities utilizing Level 2 inputs include fixed income securities, non-exchange-based derivatives, mutual funds, and fair-value hedges.
| F-14 |
Level 3. Unobservable inputs in which there is little or no market data for the asset or liability which requires the reporting entity to develop its own assumptions. Financial assets and liabilities utilizing Level 3 inputs include infrequently traded non-exchange-based derivatives and commingled investment funds and are measured using present value pricing models.
The Company determines the level in the fair value hierarchy within which each fair value measurement falls in its entirety, based on the lowest level input that is significant to the fair value measurement in its entirety. In determining the appropriate levels, the Company performs an analysis of the assets and liabilities at each reporting period end.
The carrying value of financial instruments, which consists of accounts payable and accrued expenses is considered to be representative of their respective fair values due to the short-term nature of those instruments.
Recent Accounting Pronouncements
In July 2023, the Financial Accounting Standards Board (“FASB”) issued Accounting Standards Update (“ASU”) 2023-03, Presentation of Financial Statements (Topic 205), Income Statement — Reporting Comprehensive Income (Topic 220), Distinguishing Liabilities from Equity (Topic 480), Equity (Topic 505), and Compensation — Stock Compensation (Topic 718) (“ASU 2023-03”). ASU 2023-03 amends the FASB Accounting Standards Codification to include Amendments to SEC Paragraphs pursuant to SEC Staff Accounting Bulletin No. 120, SEC Staff Announcement at the March 24, 2022 EITF Meeting, and SEC Staff Accounting Bulletin Topic 6.B, Accounting Series Release 280 — General Revision of Regulation S-X: Income or Loss Applicable to Common Stock. As ASU 2023-03 did not provide any new guidance, there was no transition or effective date associated with its adoption. The Company adopted ASU 2023-03 immediately upon its issuance in July 2023. The adoption of ASU 2023-03 did not have any impact on the Company’s consolidated financial statement presentation and related disclosures.
In November 2023, the FASB issued ASU 2023-07, Segment Reporting (Topic 280): Improvements to Reportable Segment Disclosure. ASU 2023-07 amends the FASB Accounting Standards Codification to require additional reportable segment disclosures of a public entity by requiring disclosure of significant segment expenses that are regularly provided to the chief operating decision maker, requiring other new disclosures, and requiring enhanced interim disclosures. ASU 2023-07 requires public entities with a single reportable segment to provide all the disclosures required by ASU 2023-07 and all existing segment disclosures in Topic 280 on an interim and annual basis. ASU 2023-07 is effective for annual periods beginning after December 15, 2023, and interim periods beginning after December 15, 2024, and is applied retrospectively. The Company adopted ASU 2023-07 effective January 1, 2024 for the 2024 annual period on a retrospective basis. The adoption of ASU 2023-07 resulted in additional required segment-related disclosures (see Note 3).
In November 2024, the FASB issued ASU 2024-03, Income Statement – Reporting Comprehensive Income – Expense Disaggregation Disclosures (Subtopic 220-40). ASU 2024-03 amends the FASB Accounting Standards Codification to require specified information about certain costs and expenses in the notes to the financial statements at each interim and annual reporting period, including disclosure of the amounts of purchases of inventory; employee compensation; depreciation; intangible asset amortization; and depreciation, depletion, and amortization included in each relevant expense caption on the face of the income statement within continuing operations that contains any of the expense categories previously listed. Disclosure will also be required of the total amount of selling expenses and an entity’s definition of selling expenses in annual reporting periods. ASU 2024-03 does not change or remove current expense disclosure requirements, but does affect where and how this information is presented in the notes to the financial statements. ASU 2024-03 is effective for annual reporting periods beginning January 1, 2027, and interim periods within annual reporting periods beginning January 1, 2028. Early adoption is permitted. The Company is in the process of evaluating ASU 2024-03 to determine its impact on the Company’s consolidated financial statement presentation and related disclosures.
Management does not believe that any other recently issued, but not yet effective, authoritative guidance, if currently adopted, would have a material impact on the Company’s financial statements, including their presentation and related disclosures.
| F-15 |
Reclassifications
As
a result of the adoption of ASU 2023-07 effective January 1, 2024, certain reclassifications have been made to the prior year statement
of operations to conform it to the current year presentation. In presenting general and administrative costs on the Company’s consolidated
statement of operations for the year ended December 31, 2023, $
3. Segment Information
The Company’s chief operating decision maker (“CODM”) has been identified as the Company’s President and Chief Executive Officer (“CEO”). The Company’s CODM evaluates performance and makes operating decisions about allocating resources based on financial data presented on a consolidated basis. Because the CODM evaluates financial performance on a consolidated basis, the Company has determined that it has a single operating segment composed of the consolidated financial results of the Company.
The following table presents the significant segment expenses (10% or greater) and other segment items regularly reviewed by the Company’s CODM and included in general and administrative costs.
| Years Ended December 31, | ||||||||
| 2024 | 2023 | |||||||
| Compensation to related parties: | ||||||||
| Cash-based | $ | $ | ||||||
| Stock-based | ||||||||
| Patent and licensing legal and filing fees and costs | ||||||||
| Other consulting and professional fees | ||||||||
| Insurance expense | ||||||||
| Other costs and expenses, net | ||||||||
| Total general and administrative costs | $ | $ | ||||||
The following table presents the significant segment expenses (10% or greater) and other segment items regularly reviewed by the Company’s CODM, and included in research and development costs.
| Years Ended December 31, | ||||||||
| 2024 | 2023 | |||||||
| Clinical and related oversight costs | $ | $ | ||||||
| Preclinical research focused on development of additional novel anti-cancer compounds | ||||||||
| Regulatory service costs | ||||||||
| Total research and development costs | $ | $ | ||||||
The following table presents a summary of research and development costs for the years ended December 31, 2024 and 2023 based on the respective geographical regions where such costs were incurred.
| Years Ended December 31, | ||||||||
| 2024 | 2023 | |||||||
| United States | $ | $ | ||||||
| Spain | ||||||||
| China | ||||||||
| Netherlands | ||||||||
| Total | $ | $ | ||||||
| F-16 |
The following table presents the Company’s total assets by segment at December 31, 2024 and 2023.
| December 31, | ||||||||
| 2024 | 2023 | |||||||
| Research and development assets | $ | $ | ||||||
| Corporate assets | ||||||||
| Total assets | $ | $ | ||||||
4. Stockholders’ Equity
Preferred Stock
The
Company is authorized to issue a total of shares of preferred stock, par value $ per share. On March 17, 2015, the Company
filed a Certificate of Designations, Preferences, Rights and Limitations of its Series A Convertible Preferred Stock with the Delaware
Secretary of State to amend the Company’s certificate of incorporation. The Company has designated a total of shares as
Series A Convertible Preferred Stock, which are non-voting and are not subject to increase without the written consent of a majority
of the holders of the Series A Convertible Preferred Stock or as otherwise set forth in the Preferences, Rights and Limitations. The
holders of each tranche of shares of the Series A Convertible Preferred Stock are entitled to receive a per share dividend equal
to
Based on the attributes of the Series A Convertible Preferred Stock as previously described, the Company has accounted for the Series A Convertible Preferred Stock as a permanent component of stockholders’ equity.
Common Stock
The Company is authorized to issue a total of shares of common stock, par value $ per share. As of December 31, 2024 and 2023, the Company had shares of common stock issued and outstanding.
On
June 2, 2023, the Company effected a
| F-17 |
Effective
March 10, 2023, the Company issued shares of common stock upon the exercise of a stock option in the form of a warrant held by
a consultant to the Company for shares exercisable at $
Effective
July 20, 2023, the Company sold shares of common stock at a price of $ per share and pre-funded warrants to purchase
During
the period from July 24, 2023 through August 7, 2023, the
In
a concurrent private placement to the institutional investor, the Company also sold warrants to purchase
The
registered direct offering and the concurrent private placement generated gross proceeds of $
The
exercise prices of the warrants issued to the institutional investor (exercisable at $
If such fundamental transaction is not within the Company’s control, including not being approved by the Company’s Board of Directors, the warrant holder would only be entitled to receive the same type or form of consideration (and in the same proportion) equal to the Black-Scholes valuation amount of the remaining unexercised portion of the warrant on the date of consummation of such fundamental transaction as the holders of the Company’s common stock receive. Accordingly, these warrants are classified as a component of permanent stockholders’ equity. The Company will account for any cash payment for a warrant redemption as a distribution from stockholders’ equity, as and when a fundamental transaction is consummated and such cash payment is required to be made.
| F-18 |
Common Stock Warrants
A summary of common stock warrant activity, including warrants to purchase common stock that were issued in conjunction with the Company’s public offering, during the years ended December 31, 2024 and 2023 is presented below.
| Number of Shares |
Weighted Average Exercise Price |
Weighted Average Remaining Contractual Life (in Years) |
||||||||||
| Warrants outstanding at December 31, 2022 | $ | |||||||||||
| Issued | ||||||||||||
| Exercised | ||||||||||||
| Expired | ||||||||||||
| Warrants outstanding at December 31, 2023 | $ | |||||||||||
| Issued | ||||||||||||
| Exercised | ||||||||||||
| Expired | ||||||||||||
| Warrants outstanding at December 31, 2024 | $ | |||||||||||
| Warrants exercisable at December 31, 2023 | $ | |||||||||||
| Warrants exercisable at December 31, 2024 | $ | |||||||||||
At December 31, 2024, the outstanding warrants are exercisable at the following prices per common share:
| Exercise Prices | Warrants Outstanding (Shares) |
|||||
| $ | ||||||
| $ | ||||||
| $ | ||||||
| $ | ||||||
| $ | ||||||
The
warrants exercisable at $ per share at December 31, 2024 consist of
Based on the closing fair market value of $ per share on December 31, 2024, there was no intrinsic value attributed to exercisable but unexercised common stock warrants at December 31, 2024.
Information with respect to the issuance of common stock in connection with various stock-based compensation arrangements is provided at Note 6.
5. Related Party Transactions
Related party transactions include transactions with the Company’s officers, directors and affiliates.
| F-19 |
Employment Agreements with Officers
During July and August 2020, the Company entered into one-year employment agreements with each of its executive officers at that time, consisting of Dr. John S. Kovach, Eric J. Forman, Dr. James S. Miser, and Robert N. Weingarten, payable monthly, as described below. These employment agreements were automatically renewable for additional one-year periods unless terminated by either party upon 60 days written notice prior to the end of the applicable one-year period, or by death, or by termination for cause. Except as noted below, these employment agreements were automatically renewed for additional one-year periods in July and August 2021, 2022, 2023 and 2024.
The
Company entered into an employment agreement with Dr. Kovach dated July 15, 2020, effective October 1, 2020, to provide for Dr. Kovach
to continue to act as the Company’s President, Chief Executive Officer and Chief Scientific Officer, with an annual salary of $
The
Company entered into an employment agreement with Dr. James S. Miser, M.D., effective August 1, 2020, to act as the Company’s Chief
Medical Officer, with an annual salary of $
The
Company entered into an employment agreement with Eric J. Forman effective July 15, 2020, as amended on August 12, 2020, to act as the
Company’s Chief Administrative Officer, with an annual salary of $
The
Company entered into an employment agreement with Robert N. Weingarten effective August 12, 2020 to act as the Company’s Vice President
and Chief Financial Officer, with an annual salary of $
The
Company entered into an employment agreement with Bastiaan van der Baan effective September 26, 2023 to act as the Company’s President
and Chief Executive Officer and as Vice Chairman of the Board of Directors, with an annual salary of $
| F-20 |
On
May 31, 2024, the Company entered into a consulting agreement with Dr. Jan H.M. Schellens, M.D., Ph.D. Pursuant to the agreement, effective
July 1, 2024, the Company engaged Dr. Schellens as a consultant, and, effective August 1, 2024, as the Company’s Chief Medical
Officer. The term of the agreement is in effect from July 1, 2024 until the earliest of (i) termination by either party upon sixty days’
notice, (ii) Dr. Schellens’ death or disability, or (iii) termination by the Company for breach as provided in the agreement. Under
the agreement, Dr. Schellens provides his services for two days per week with the specific days in each week based on arrangements agreed
to from time to time between Dr. Schellens and the Company’s Chief Executive Officer. The Company pays Dr. Schellens an annual
compensation of Euros (approximately $ as of December 31, 2024), payable on a monthly basis. During the year ended December
31, 2024, the Company paid $
Effective
as of June 15, 2022, Dr. René Bernards was appointed to the Company’s Board of Directors as an independent director. Dr.
Bernards is a leader in the field of molecular carcinogenesis and is employed by the Netherlands Cancer Institute in Amsterdam. Upon
his appointment, it was agreed that Dr. Bernards would receive annual compensation for his services on the Board only in the form of
cash, in lieu of the annual June 30 grant of stock options as provided to the Company’s other non-officer directors. During the
years ended December 31, 2024 and 2023, the Company recorded charges to general and administrative costs in the consolidated statement
of operations of $
In conjunction with the Company’s efforts to preserve cash, effective with the quarter ended June 30, 2024, Dr. Bernards agreed to receive equity-based compensation for his services on the Board, for the quarters ended June 30, 2024, September 30, 2024 and December 31, 2024. In order to reconcile his Board compensation with that of the other non-officer directors, Dr. Bernards has agreed to receive the same Board compensation, both in form and amount, as the other non-officer directors.
Previously, on October 8, 2021, the Company had entered into a Development Collaboration Agreement (subsequently amended and extended) with the Netherlands Cancer Institute, Amsterdam, one of the world’s leading comprehensive cancer centers, and Oncode Institute, Utrecht, a major independent cancer research center, to identify the most promising drugs to be combined with LB-100, and potentially LB-100 analogues, to be used to treat a range of cancers, as well as to identify the specific molecular mechanisms underlying the identified combinations (see Note 8).
Compensatory Arrangements for Members of the Board of Directors
Effective April 9, 2021, the Board of Directors approved a comprehensive cash and equity compensation program for the non-officer directors for their services on the Board of Directors (the “Board Plan”), which was subsequently amended effective May 25, 2022 and July 9, 2024. Officers who also serve on the Board of Directors are not compensated separately for their service on the Board of Directors.
Cash compensation for directors, payable quarterly, is as follows:
Base
director compensation - $
Chairman
of audit committee – additional $
Chairman
of any other committees – additional $
Member
of audit committee – additional $
Member
of any other committees – additional $
In conjunction with the Company’s efforts to preserve cash, the Board approved an amendment to the Board Plan, such that for the quarters ended June 30, 2024, September 30, 2024 and December 31, 2024, the non-officer directors (including Dr. Bernards) received, in lieu of cash compensation, stock options exercisable for a period of five years, vesting immediately, to purchase common stock at an exercise price based on the closing market price upon issuance, with the amount of such stock options equal to the cash payment such director would otherwise have been entitled to receive for such quarter, divided by their quarterly value as determined pursuant to the Black-Scholes option-pricing model. The Board may extend this amendment to the Board Plan for additional quarterly periods subsequent to December 31, 2024.
| F-21 |
Equity compensation for directors is as follows:
Appointment
of new directors – The Company grants options to purchase shares of common stock, exercisable for a period of ,
at the closing market price on the date of grant, vesting 50% on the grant date and the remaining % vesting % on the last day of
each calendar quarter beginning in the quarter immediately subsequent to the date of the grant until fully vested, subject to continued
service. At the discretion of the Board of Directors, for a nominee to the Board of Directors who is restricted by their respective institution
or employer from receiving equity-based compensation, in lieu of the grant of such stock options, the Company may elect to pay a one-time
cash fee of $
Annual
grant of options to directors – Effective on the last business day of the month of June, the Company grants options to purchase
shares of common stock, exercisable for a period of five years, at the closing market price on the date of grant, vesting %
on the last day of each calendar quarter beginning in the quarter immediately subsequent to the date of grant until fully vested, subject
to continued service. If any director has served for less than 12 full calendar months on the grant date, the amount of such stock option
grant is prorated based on the length of service of such director. At the discretion of the Board of Directors, for a nominee to the
Board of Directors who is restricted by their respective institution or employer from receiving equity-based compensation, in lieu of
the grant of such stock options, the Company may elect to pay an annual cash fee of $
Total cash compensation paid to non-officer directors was $ and $, respectively, for the years ended December 31, 2024 and 2023.
Stock-based compensation granted to members of the Company’s Board of Directors, officers and affiliates is described at Note 6.
A summary of related party costs, including compensation under employment and consulting agreements and fees paid to non-officer directors for their services on the Board of Directors, for the years ended December 31, 2024 and 2023, is presented below.
Years Ended December 31, |
||||||||
| 2024 | 2023 | |||||||
| Related party costs: | ||||||||
| Cash-based | $ | $ | ||||||
| Stock-based | ||||||||
| Total | $ | $ | ||||||
The Company periodically issues common stock and stock options as incentive compensation to directors and as compensation for the services of employees, contractors, and consultants of the Company.
On July 14, 2020, the Board of Directors of the Company adopted the 2020 Stock Incentive Plan (the “2020 Plan”), which was subsequently approved by the stockholders of the Company. The 2020 Plan provides for the granting of equity-based awards, consisting of stock options, restricted stock, restricted stock units, stock appreciation rights, and other stock-based awards to employees, officers, directors and consultants of the Company and its affiliates, initially for a total of shares of the Company’s common stock, under terms and conditions as determined by the Company’s Board of Directors. On October 7, 2022, the stockholders of the Company approved an amendment to the 2020 Plan to increase the number of common shares issuable thereunder by shares, to a total of shares. On November 27, 2023, the stockholders of the Company approved an amendment to the 2020 Plan to increase the number of common shares issuable thereunder by shares, to a total of shares.
| F-22 |
As of December 31, 2024, unexpired stock options for shares were issued and outstanding under the 2020 Plan and shares were available for issuance under the 2020 Plan.
The fair value of a stock option award is calculated on the grant date using the Black-Scholes option-pricing model. The risk-free interest rate is based on the U.S. Treasury yield curve in effect as of the grant date. The expected dividend yield assumption is based on the Company’s expectation of dividend payouts and is assumed to be zero. The estimated volatility is based on the historical volatility of the Company’s common stock, calculated utilizing a look-back period approximately equal to the contractual life of the stock option being granted. Unless sufficient historical exercise data is available, the expected life of the stock option is calculated as the mid-point between the vesting period and the contractual term (the “simplified method”). The fair market value of the common stock is determined by reference to the quoted market price of the common stock on the grant date.
| Risk-free interest rate | % to | % | ||
| Expected dividend yield | % | |||
| Expected volatility | % to | % | ||
| Expected life | to years |
For stock options requiring an assessment of value during the year ended December 31, 2023, the fair value of each stock option award was estimated using the Black-Scholes option-pricing model with the following assumptions:
| Risk-free interest rate | % to | % | ||
| Expected dividend yield | % | |||
| Expected volatility | % | |||
| Expected life | years |
On
July 15, 2020, as amended on August 12, 2020, in connection with the employment agreement with Eric J. Forman, Mr. Forman was granted
stock options to purchase shares of the Company’s common stock. The options can be exercised on a cashless basis. The options
are exercisable for a period of at an exercise price of $ per share, which was equal to the closing market price of the
Company’s common stock on the grant date. The fair value of these stock options, as calculated pursuant to the Black-Scholes option-pricing model,
was determined to be $
On
August 1, 2020, in connection with an employment agreement with Dr. James S. Miser, M.D., Dr. Miser was granted stock options to purchase
shares of the Company’s common stock. The options can be exercised on a cashless basis. The options are exercisable for a
period of at an exercise price of $ per share, which was equal to the closing market price of the Company’s common
stock on the effective date of the employment agreement. The fair value of these stock options, as calculated pursuant to the Black-Scholes option-pricing
model, was determined to be $
| F-23 |
On
August 12, 2020, in connection with the employment agreement with Robert N. Weingarten, Mr. Weingarten was granted stock options to purchase
shares of the Company’s common stock. The options can be exercised on a cashless basis. The options are exercisable for a
period of at an exercise price of $ per share, which was equal to the closing market price of the Company’s common
stock on the grant date. The fair value of these stock options, as calculated pursuant to the Black-Scholes option-pricing model, was determined to
be $
On
May 11, 2021, the Board of Directors appointed Regina Brown to the Board of Directors. In connection with her appointment to the Board
of Directors, and in accordance with the Company’s cash and equity compensation package for members of the Board of Directors,
Ms. Brown was granted stock options to purchase shares of the Company’s common stock, exercisable for a period of
at an exercise price of $ per share (the closing market price on the grant date), The fair value of these stock options, as calculated
pursuant to the Black-Scholes option-pricing model, was determined to be $
On
June 30, 2021, the Board of Directors, in accordance with the Company’s cash and equity compensation package for members of the
Board of Directors, granted to each of the five non-officer directors of the Company stock options to purchase shares (a total
of shares) of the Company’s common stock, exercisable for a period of at an exercise price of $ per share
(the closing market price on the grant date),
The fair value of these stock options, as calculated pursuant to the Black-Scholes option-pricing model, was determined to be $
On
June 17, 2022, the Board of Directors appointed Bas van der Baan to the Board of Directors. In connection with his appointment to the
Board of Directors, and in accordance with the Company’s cash and equity compensation package for members of the Board of Directors,
Mr. van der Baan was granted stock options to purchase shares of the Company’s common stock, exercisable for a period of
at an exercise price of $ per share (the closing market price on the grant date), The
fair value of these stock options, as calculated pursuant to the Black-Scholes option-pricing model, was determined to be $
On
June 30, 2022, the Board of Directors, in accordance with the Company’s cash and equity compensation package for members of the
Board of Directors, granted to each of the five non-officer directors of the Company stock options to purchase shares (a total
of shares) of the Company’s common stock, exercisable for a period of at an exercise price of $ per share
(the closing market price on the grant date), The fair value of these stock options, as calculated pursuant to the Black-Scholes option-pricing model,
was determined to be $
| F-24 |
On
November 6, 2022, the Board of Directors granted to each of the four officers of the Company stock options to purchase shares
(a total of shares) of the Company’s common stock, exercisable for a period of at an exercise price of $
per share, The
total fair value of the stock options, as calculated pursuant to the Black-Scholes option-pricing model, was determined to be
$
On
June 30, 2023, the Board of Directors, in accordance with the Company’s cash and equity compensation package for members of the
Board of Directors, granted to each of the four non-officer directors of the Company stock options to purchase shares (a total
of shares) of the Company’s common stock, exercisable for a period of at an exercise price of $ per share
(the closing market price on the grant date), The fair value of these stock options, as calculated pursuant to the Black-Scholes option-pricing model,
was determined to be $
On
September 26, 2023, in connection with the employment agreement entered into with Bas van der Baan, Mr. van der Baan was granted stock
options to purchase shares of the Company’s common stock. The options can be exercised on a cashless basis. The options
are exercisable for a period of at an exercise price of $ per share, which was equal to the closing market price of the
Company’s common stock on the grant date. The fair value of these stock options,
as calculated pursuant to the Black-Scholes option-pricing model, was determined to be $
On
June 30, 2024, the Board of Directors, in accordance with the Company’s cash and equity compensation package for members of the
Board of Directors, granted to each of the four non-officer directors of the Company stock options to purchase shares (a total
of shares) of the Company’s common stock, exercisable for a period of at an exercise price of $ per share
(the closing market price on the grant date), The fair value of these stock options, as calculated pursuant to the Black-Scholes option-pricing model,
was determined to be $
On
June 30, 2024, the Board of Directors, in conjunction with the Company’s efforts to preserve cash, granted to the four non-officer
directors of the Company a total of stock options to purchase shares of the Company’s common stock, exercisable for a period
of at an exercise price of $ per share (the closing market price on the grant date) The stock options were granted in
lieu of cash compensation, are exercisable for a period of and were immediately vested. The number of stock options granted
to each of the four non-officer directors of the Company was equal to the cash payment such director would otherwise have been entitled
to receive for the quarter ended June 30, 2024, divided by their quarterly value as determined pursuant to the Black-Scholes option-pricing
model, and was determined to be $
| F-25 |
On
July 1, 2024, in connection with the consulting agreement with Dr. Jan H.M. Schellens, M.D., Ph.D., Dr. Schellens was granted stock options
to purchase shares of the Company’s common stock. The options can be exercised on a cashless basis. The options are exercisable
for a period of at an exercise e price of $ per share, which was equal to the closing market price of the Company’s
common stock on the grant date. The fair value of these stock options, as calculated pursuant to the Black-Scholes option-pricing model,
was determined to be $
On
September 30, 2024, the Board of Directors, in conjunction with the Company’s efforts to preserve cash, granted to the four non-officer
directors of the Company a total of stock options to purchase shares of the Company’s common stock, exercisable for a period
of at an exercise price of $ per share (the closing market price on the grant date) The stock options were granted in
lieu of cash compensation, are exercisable for a period of and were immediately vested. The number of stock options granted
to each of the four non-officer directors of the Company was equal to the cash payment such director would otherwise have been entitled
to receive for the quarter ended September 30, 2024, divided by their quarterly value as determined pursuant to the Black-Scholes option-pricing
model, and was determined to be $
On
January 20, 2025, the Board of Directors, in conjunction with the Company’s efforts to preserve cash, granted to the four non-officer
directors of the Company a total of stock options to purchase shares of the Company’s common stock, exercisable for a period
of at an exercise price of $ per share (the closing market price on the grant date) The stock options were granted in
lieu of cash compensation, are exercisable for a period of and were immediately vested. The number of stock options granted
to each of the four non-officer directors of the Company was equal to the cash payment such director would otherwise have been entitled
to receive for the quarter ended December 31, 2024, divided by their grant date value as determined pursuant to the Black-Scholes option-pricing
model, and was determined to be $
Dr. Philip Palmedo, a director of the Company since 2006, did not stand for re-election to the Company’s Board of Directors at the Company’s annual meeting of stockholders held on October 7, 2022. Gil Schwartzberg, a former director of the Company, died on October 30, 2022. Dr. John S. Kovach, the Chairman of the Board of Directors and the Company’s President and Chief Executive Officer, and Chief Scientific Officer, died on October 5, 2023, the employment agreement of the Company’s Chief Medical Officer, Dr. James S. Miser expired on July 31, 2024, and the employment agreement of the Company’s Vice President and Chief Operating Officer, Eric J. Forman, terminated upon his resignation from the Company on December 31, 2024. Accordingly, the unvested stock options for each such person ceased vesting effective as of the respective dates that their services to the Company terminated. Furthermore, the expiration date of all vested stock options owned by each such person contractually expire one year from the respective dates that their services to the Company terminate.
| Years Ended | ||||||||
| December 31, | ||||||||
| 2024 | 2023 | |||||||
| Related parties | $ | $ | ||||||
| Non-related parties | ||||||||
| Total stock-based compensation costs | $ | $ | ||||||
| F-26 |
| Number of Shares |
Weighted Average Exercise Price |
Weighted Average Remaining Contractual Life (in Years) |
||||||||||
| Stock options outstanding at December 31, 2022 | $ | |||||||||||
| Granted | ||||||||||||
| Exercised | ( |
) | ||||||||||
| Expired | ( |
) | ||||||||||
| Stock options outstanding at December 31, 2023 | ||||||||||||
| Granted | ||||||||||||
| Exercised | ||||||||||||
| Expired | ( |
) | ||||||||||
| Stock options outstanding at December 31, 2024 | $ | |||||||||||
| Stock options exercisable at December 31, 2023 | $ | |||||||||||
| Stock options exercisable at December 31, 2024 | $ | |||||||||||
Total
deferred compensation expense for the outstanding value of unvested stock options was approximately $
| Exercise Prices | Options Outstanding (Shares) |
Options Exercisable (Shares) |
||||||||
| $ | ||||||||||
| $ | ||||||||||
| $ | ||||||||||
| $ | ||||||||||
| $ | ||||||||||
| $ | ||||||||||
| $ | ||||||||||
| $ | ||||||||||
| $ | ||||||||||
| $ | ||||||||||
| $ | ||||||||||
| $ | ||||||||||
| $ | ||||||||||
| $ | ||||||||||
| $ | ||||||||||
Based on the closing fair market value of $ per share on December 31, 2024, the intrinsic value attributed to exercisable but unexercised common stock options was approximately $ at December 31, 2024.
Outstanding stock options to acquire shares of the Company’s common stock had not vested at December 31, 2024.
| F-27 |
Upon the exercise of such stock options, the Company expects to satisfy the related stock obligations through the issuance of authorized but unissued shares of common stock.
7. Income Taxes
Deferred income taxes reflect the net tax effects of temporary differences between the carrying amounts of assets and liabilities for financial reporting purposes and the amounts used for income tax purposes. Significant components of the Company’s deferred tax assets as of December 31, 2024 and 2023 are as follows:
| December 31, | ||||||||
| 2024 | 2023 | |||||||
| Research credits | $ | $ | ||||||
| Capitalized research and development | ||||||||
| Stock-based compensation | ||||||||
| Net operating loss carryforwards | ||||||||
| Total deferred tax assets | ||||||||
| Valuation allowance | ( |
) | ( |
) | ||||
| Net deferred tax assets | $ | $ | ||||||
In assessing the potential realization of deferred tax assets, management considers whether it is more likely than not that some portion or all of the deferred tax assets will be realized. The ultimate realization of deferred tax assets is dependent upon the Company attaining future taxable income during the periods in which those temporary differences become deductible. As of December 31, 2024 and 2023, management was unable to determine if it is more likely than not that the Company’s deferred tax assets will be realized and has therefore recorded an appropriate valuation allowance against deferred tax assets at such dates.
| Years Ended December 31, | ||||||||
| 2024 | 2023 | |||||||
| U. S. federal statutory tax rate | ( |
)% | ( |
)% | ||||
| State income taxes, net of federal tax benefit | ( |
)% | ( |
)% | ||||
| Expirations related to stock-based compensation | % | % | ||||||
| Adjustment to deferred tax asset | ( |
)% | ( |
)% | ||||
| Change in valuation allowance | % | % | ||||||
| Effective tax rate | % | % | ||||||
At
December 31, 2024, the Company has available net operating loss carryforwards for federal and state income tax purposes of approximately
$
The
state net operating loss carryovers include approximately $
| F-28 |
In addition, under Section 382 of the Internal Revenue Code of 1986, as amended, and certain corresponding provisions of state law, if a corporation undergoes an “ownership change”, which is generally defined as a greater than 50% change, by value, in the ownership of its equity over a three-year period, the corporation’s ability to use its pre-change NOL carryforwards and other pre-change tax attributes to offset its post-change income might be limited.
As the Company’s net operating losses have yet to be utilized, all previous tax years since 2006 remain subject to adjustment by Federal authorities and other jurisdictions in which the Company currently operates or has operated in the past.
8. Commitments and Contingencies
Legal Claims
The Company may be subject to legal claims and actions from time to time as part of its business activities. As of December 31, 2024 and 2023, the Company was not subject to any threatened or pending lawsuits, legal claims or legal proceedings.
Principal Commitments
Clinical Trial Agreements
At
March 14, 2025, the Company’s remaining financial contractual commitments pursuant to clinical trial agreements and clinical trial
monitoring agreements not yet incurred, as described below, aggregated $
| F-29 |
The following is a summary of the Company’s ongoing contractual clinical trials described below as of March 14, 2025:
| Description of Clinical Trial | Institution | Start Date | Projected End Date |
Number of Patients in Trial |
Study Objective | Clinical Update |
Expected Date of Preliminary Efficacy Signal |
NCT No. |
Remaining Financial Contractual Commitment |
|||||||||||||
| Netherlands Cancer Institute (NKI) | Determine RP2D with atezolizumab | First patient entered August 2024, in total two patients entered | NCT06012734 | (1 | ) | |||||||||||||||||
| GEIS | Determine MTD and RP2D | Fourteen patients entered | NCT05809830 | $ | ||||||||||||||||||
| GEIS | TBD | TBD | Determine efficacy: PFS | Clinical trial not yet begun (subject to completion of Phase 1b GEIS clinical trial) | TBD | NCT05809830 | $ | (1 | ) | |||||||||||||
| MD Anderson | Determine the OS of patients with recurrent ovarian clear cell carcinoma | Nine patients entered | NCT06065462 | (1 | ) | |||||||||||||||||
| Total | $ | |||||||||||||||||||||
| (1) |
Netherlands Cancer Institute. Effective June 10, 2024, the Company entered into a Clinical Trial Agreement with the Netherlands Cancer Institute (“NKI”) (see Note 5) to conduct a Phase 1b clinical trial of the Company’s protein phosphatase inhibitor, LB-100, combined with atezolizumab, a PD-L1 inhibitor, the proprietary molecule of F. Hoffman-La Roche Ltd. (“Roche”), for patients with microsatellite stable metastatic colorectal cancer. Under the agreement, the Company will provide its lead compound, LB-100, and under a separate agreement between NKI and Roche, Roche will provide atezolizumab and financial support for the clinical trial. The Company has no obligation to and will not provide any reimbursement of clinical trial costs. Pursuant to the agreement and the protocol set forth in the agreement, the clinical trial will be conducted by NKI at NKI’s site in Amsterdam by principal investigator Neeltje Steeghs, MD, PhD, and NKI will be responsible for the recruitment of patients. The agreement provides for the protection of the respective intellectual property rights of each of the Company, NKI and Roche.
This Phase 1b clinical trial will evaluate safety, optimal dose and preliminary efficacy of LB-100 combined with atezolizumab for the treatment of patients with metastatic microsatellite stable colorectal cancer. Immunotherapy using monoclonal antibodies like atezolizumab can enhance the body’s immune response against cancer and hinder tumor growth and spread. LB-100 has been found to improve the effectiveness of anticancer drugs in killing cancer cells by inhibiting a protein called PP2A on cell surfaces. Blocking PP2A increases stress signals in tumor cells expressing the PP2A protein. Accordingly, combining atezolizumab with LB-100 may enhance treatment efficacy for metastatic colorectal cancer, as cancer cells with heightened stress signals are more vulnerable to immunotherapy.
This study comprises a dose escalation phase and a dose expansion phase. The objective of the dose escalation phase is to determine the recommended Phase 2 dose (RP2D) of LB-100 when combined with the standard dosage of atezolizumab. The dose expansion phase will further investigate the preliminary efficacy, safety, tolerability, and pharmacokinetics/dynamics of the LB-100 and atezolizumab combination. The clinical trial opened in August 2024 with the enrollment of the first patient. A total of two patients have been enrolled to date. Patient accrual is expected to take up to 24 months, with a maximum of 37 patients with advanced colorectal cancer to be enrolled in this study.
The principal investigator of the colorectal study testing LB-100 in combination with atezolizumab is currently investigating two Serious Adverse Events (“SAEs”) observed in the clinical trial. The Investigational Review Board (IRB) of the Netherlands Cancer Institute has requested additional information with respect to these SAEs and the study has been paused for enrollment until the IRB’s questions have been satisfactorily addressed (see “Specific Risks Associated with the Company’s Business Activities - Serious Adverse Events” below for additional information).
| F-30 |
The Company has no financial contractual commitment associated with this clinical trial.
City of Hope. Effective January 18, 2021, the Company executed a Clinical Research Support Agreement (the “Agreement”) with the City of Hope National Medical Center, an NCI-designated comprehensive cancer center, and City of Hope Medical Foundation (collectively, “City of Hope”), to carry out a Phase 1b clinical trial of LB-100, the Company’s first-in-class protein phosphatase inhibitor, combined with an FDA-approved standard regimen for treatment of untreated extensive-stage disease small cell lung cancer (“ED-SCLC”). LB-100 was given in combination with carboplatin, etoposide and atezolizumab, an FDA-approved standard of care regimen, to previously untreated ED-SCLC patients. The LB-100 dose was to be escalated with the standard fixed doses of the 3-drug regimen to reach a recommended Phase 2 dose (“RP2D”). Patient entry was to be expanded so that a total of 12 patients would be evaluable at the RP2D to confirm the safety of the LB-100 combination and to look for potential therapeutic activity as assessed by objective response rate, duration of overall response, progression-free survival, and overall survival.
The clinical trial was initiated on March 9, 2021, with patient accrual expected to take approximately two years to complete. Because patient accrual was slower than expected, effective March 6, 2023, the Company and City of Hope added the Sarah Cannon Research Institute (“SCRI”), Nashville, Tennessee, to the ongoing Phase 1b clinical trial. The Company and City of Hope continued efforts to increase patient accrual by adding additional sites and by modifying the protocol to increase the number of patients eligible for the clinical trial. The impact of these efforts to increase patient accrual and to decrease time to completion was evaluated in subsequent quarters.
After
evaluating patient accrual through June 30, 2024, the Company and City of Hope agreed to close the clinical trial. Pursuant to the terms
of the Agreement, the Company provided notice to City of Hope of the Company’s intent to terminate the Agreement effective as of
July 8, 2024. Upon closure, the Company incurred a prorated charge of $
During
the year ended December 31, 2024 and 2023, the Company incurred costs of $
GEIS. Effective July 31, 2019, the Company entered into a Collaboration Agreement for an Investigator-Initiated Clinical Trial with the Spanish Sarcoma Group (Grupo Español de Investigación en Sarcomas or “GEIS”), Madrid, Spain, to carry out a study entitled “Randomized phase I/II trial of LB-100 plus doxorubicin vs. doxorubicin alone in first line of advanced soft tissue sarcoma”. The purpose of this clinical trial is to obtain information with respect to the efficacy and safety of LB-100 combined with doxorubicin in soft tissue sarcomas. Doxorubicin is the global standard for initial treatment of advanced soft tissue sarcomas (“ASTS”). Doxorubicin alone has been the mainstay of first line treatment of ASTS for over 40 years, with little improvement in survival from adding cytotoxic compounds to or substituting other cytotoxic compounds for doxorubicin. In animal models, LB-100 consistently enhances the anti-tumor activity of doxorubicin without apparent increases in toxicity.
GEIS has a network of referral centers in Spain and across Europe that have an impressive track record of efficiently conducting innovative studies in ASTS. The Company agreed to provide GEIS with a supply of LB-100 to be utilized in the conduct of this clinical trial, as well as to provide funding for the clinical trial. The goal is to enter approximately 150 to 170 patients in this clinical trial over a period of two to four years. The Phase 1 portion of the study began in the quarter ended June 30, 2023 to determine the recommended Phase 2 dose of the combination of doxorubicin and LB-100. As advanced sarcoma is a very aggressive disease, the design of the Phase 2 portion of the study assumes a median progression-free survival (“PFS”), no evidence of disease progression or death from any cause, of 4.5 months in the doxorubicin arm and an alternative median PFS of 7.5 months in the doxorubicin plus LB-100 arm to demonstrate a statistically significant decrease in relative risk of progression or death by adding LB-100. There is a planned interim analysis of the primary endpoint when approximately 50% of the 102 events required for final analysis is reached.
| F-31 |
The Company had previously expected that this clinical trial would commence during the quarter ended June 30, 2020. However, during July 2020, the Spanish regulatory authority advised the Company that although it had approved the scientific and ethical basis of the protocol, it required that the Company manufacture new inventory of LB-100 under current Spanish pharmaceutical manufacturing standards. These standards were adopted subsequent to the production of the Company’s existing LB-100 inventory.
In order to manufacture a new inventory supply of LB-100 for the GEIS clinical trial, the Company engaged a number of vendors to carry out the multiple tasks needed to make and gain approval of a new clinical product for investigational study in Spain. These tasks included the synthesis under good manufacturing practice (GMP) of the active pharmaceutical ingredient (API), with documentation of each of the steps involved by an independent auditor. The API was then transferred to a vendor that prepares the clinical drug product, also under GMP conditions documented by an independent auditor. The clinical drug product was then sent to a vendor to test for purity and sterility, provide appropriate labels, store the drug, and distribute the drug to the clinical centers for use in the clinical trials. A formal application documenting all steps taken to prepare the clinical drug product for clinical use was submitted to the appropriate regulatory authorities for review and approval before being used in a clinical trial.
As
of December 31, 2024, this program to provide new inventory of the clinical drug product for the Spanish Sarcoma Group study, and potentially
for subsequent multiple trials within the European Union, had cost approximately $
On October 13, 2022, the Company announced that the Spanish Agency for Medicines and Health Products (Agencia Española de Medicamentos y Productos Sanitarios or “AEMPS”) had authorized a Phase 1b/randomized Phase 2 study of LB-100, the Company’s lead clinical compound, plus doxorubicin, versus doxorubicin alone, the global standard for initial treatment of ASTS. Consequently, this clinical trial commenced during the quarter ended June 30, 2023 and is expected to be completed and a report prepared by December 31, 2026. In April 2023, GEIS completed its first site initiation visit in preparation for the clinical trial at Fundación Jiménez Díaz University Hospital (Madrid). Up to 170 patents will be entered into the clinical trial. The recruitment for the Phase 1b portion of the protocol was extended with two patients and was completed during the quarter ended September 30, 2024. The Company expects to have data on toxicity and preliminary efficacy from this portion of the clinical trial during the quarter ending December 31, 2025.
Given
the focus on the combination of LB-100 with immunotherapy in ovarian clear cell carcinoma and colorectal cancer and the availability
of capital resources, the Company entered into Amendment No. 1 to the Collaboration Agreement effective March 11, 2025 that relieved
the Company of the financial obligation to support the randomized Phase 2 portion of the clinical trial contemplated in the Collaboration
Agreement of approximately $
The
Company’s agreement with GEIS provided for various payments based on achieving specific milestones over the term of the agreement.
During the years ended December 31, 2024 and 2023, the Company incurred costs of $
The
Company’s aggregate commitment pursuant to this agreement, less amounts previously paid to date, totaled approximately $
MD Anderson Cancer Center Clinical Trial. On September 20, 2023, the Company announced an investigator-initiated Phase 1b/2 collaborative clinical trial to assess whether adding LB-100 to a human programmed death receptor-1 (“PD-1”) blocking antibody of GSK plc (“GSK”), dostarlimab-gxly, may enhance the effectiveness of immunotherapy in the treatment of ovarian clear cell carcinoma (“OCCC”). The study objective is to determine the overall survival (“OS”) of patients with OCCC. The clinical trial is being sponsored by The University of Texas MD Anderson Cancer Center (“MD Anderson”) and is being conducted at The University of Texas - MD Anderson Cancer Center. The Company is providing LB-100 and GSK is providing dostarlimab-gxly and financial support for the clinical trial. On January 29, 2024, the Company announced the entry of the first patient into this clinical trial. The Company currently expects that this clinical trial will be completed by December 31, 2027.
| F-32 |
On February 25, 2025, the Company announced that it has added the Robert H. Lurie Comprehensive Cancer Center (Lurie Cancer Center) of Northwestern University as a second site in a clinical trial combining the Company’s proprietary compound LB-100 with GSK’s dostarlimab to treat ovarian clear cell cancer. Patient recruitment is underway, and the first patient has been dosed.
Moffitt. Effective August 20, 2018, the Company entered into a Clinical Trial Research Agreement with the Moffitt Cancer Center and Research Institute Hospital Inc., Tampa, Florida (“Moffitt”), effective for a term of five years. Pursuant to the Clinical Trial Research Agreement, Moffitt agreed to conduct and manage a Phase 1b/2 clinical trial to evaluate the toxicity and therapeutic benefit of the Company’s lead anti-cancer clinical compound LB-100 to be administered intravenously in patients with low or intermediate-1 risk myelodysplastic syndrome (“MDS”).
In November 2018, the Company received approval from the U.S. Food and Drug Administration for its Investigational New Drug (“IND”) Application to conduct a Phase 1b/2 clinical trial to evaluate the toxicity and therapeutic benefit of LB-100 in patients with low and intermediate-1 risk MDS who had failed or were intolerant of standard treatment. This Phase 1b/2 clinical trial utilized LB-100 as a single agent in the treatment of patients with low and intermediate-1 risk MDS.
The clinical trial began at a single site in April 2019 and the first patient was entered into the clinical trial in July 2019. During the year ended December 31, 2023, the clinical trial was closed. Although the maximum tolerated dose (“MTD”) was not achieved, there was no dose-limiting toxicity noted.
During
the years ended December 31, 2024 and 2023, the Company incurred costs of $
During September 2023, the Company decided not to pursue further studies in MDS, as other, more promising, opportunities had become available (see “Patent and License Agreements - Moffitt” below).
National Cancer Institute Pharmacologic Clinical Trial. In May 2019, the National Cancer Institute (“NCI”) initiated a glioblastoma (“GBM”) pharmacologic clinical trial. This study was being conducted and funded by the NCI under a Cooperative Research and Development Agreement, with the Company responsible for providing the LB-100 clinical compound.
Primary malignant brain tumors (gliomas) are very challenging to treat. Radiation combined with the chemotherapeutic drug temozolomide has been the mainstay of therapy of the most aggressive gliomas (glioblastoma multiforme or GBM) for decades, with little further benefit gained by the addition of one or more anti-cancer drugs, but without major advances in overall survival for the majority of patients. In animal models of GBM, the Company’s novel protein phosphatase inhibitor, LB-100, has been found to enhance the effectiveness of radiation, temozolomide chemotherapy treatments and immunotherapy, raising the possibility that LB-100 may improve outcomes of standard GBM treatment in the clinic. Although LB-100 has proven safe in patients at doses associated with apparent anti-tumor activity against several human cancers arising outside the brain, the ability of LB-100 to penetrate tumor tissue arising in the brain was not known. Many drugs potentially useful for GBM treatment do not enter the brain in amounts necessary for anti-cancer action.
The NCI study was designed to determine the extent to which LB-100 enters recurrent malignant gliomas. Patients having surgery to remove one or more tumors received one dose of LB-100 prior to surgery and had blood and tumor tissue analyzed to determine the amount of LB-100 present and to determine whether the cells in the tumors showed the biochemical changes expected to be present if LB-100 reached its molecular target. As a result of the innovative design of the NCI study, it was believed that data from a few patients would be sufficient to provide a sound rationale for conducting a larger clinical trial to determine the effectiveness of adding LB-100 to the standard treatment regimen for GBMs. Blood and brain tumor tissue were analyzed from seven patients after intravenous infusion of a single dose of LB-100. Results of the investigation demonstrated that there was virtually no entry of LB-100 into the brain tumor tissue. Accordingly, alternative methods of drug delivery will be required to determine if LB-100 has meaningful clinical anti-cancer activity against glioblastoma multiforme and other aggressive brain tumors.
| F-33 |
Clinical Trial Monitoring Agreements
MD Anderson Cancer Center Clinical Trial. On May 15, 2024, the Company signed a letter of intent with Theradex to monitor the MD Andersen investigator-initiated Phase 1b/2 collaborative clinical trial to assess whether adding LB-100 to a human programmed death receptor-1 (“PD-1”) blocking antibody of GSK plc (“GSK”), dostarlimab-gxly, may enhance the effectiveness of immunotherapy in the treatment of ovarian clear cell carcinoma (“OCCC”). On August 19, 2024, the Company signed a work order agreement with Theradex to monitor the MD Anderson clinical trial. The study oversight is expected to be completed by January 31, 2027.
Costs
under this letter of intent and related work order agreement are estimated to be approximately $
The
Company’s aggregate commitment pursuant to this letter of intent, less amounts previously paid to date, totaled approximately $
City
of Hope. On February 5, 2021, the Company signed a new work order agreement with Theradex to monitor the City of Hope investigator-initiated
clinical trial in small cell lung cancer in accordance with FDA requirements for oversight by the sponsoring party. Costs under this
work order agreement were estimated to be approximately $
As a result of the closure of the Agreement with City of Hope effective July 8, 2024 (see “Clinical Trial Agreements – City of Hope” above), the work order agreement with Theradex to monitor this clinical trial was concurrently terminated, although nominal oversight trailing costs subsequent to July 8, 2024 are expected to be incurred relating to the closure of this study.
GEIS. On June 22, 2023, the Company finalized a work order agreement with Theradex, to monitor the GEIS investigator-initiated clinical Phase I/II randomized trial of LB-100 plus doxorubicin vs. doxorubicin alone in first line of advanced soft tissue sarcoma. The study oversight is expected to be completed by December 31, 2026.
Costs
under this work order agreement are estimated to be approximately $
| F-34 |
The
Company’s aggregate commitment pursuant to this clinical trial monitoring agreement, less amounts previously paid to date, totaled
approximately $
Netherlands Cancer Institute. On August 27, 2024, the Company finalized a work order agreement with Theradex, to monitor the NKI Phase 1b clinical trial of LB-100 combined with atezolizumab, a PD-L1 inhibitor, for patients with microsatellite stable metastatic colorectal cancer. The study oversight is expected to be completed by May 31, 2027.
Costs
under this work order agreement are estimated to be approximately $
The
Company’s aggregate commitment pursuant to this clinical trial monitoring agreement, less amounts previously paid to date, totaled
approximately $
Patent and License Agreements
National Institute of Health. Effective February 23, 2024, the Company entered into a Patent License Agreement (the “License Agreement”) with the National Institute of Neurological Disorders and Stroke (“NINDS”) and the National Cancer Institute (“NCI”), each an institute or center of the National Institute of Health (“NIH”). Pursuant to the License Agreement, the Company has licensed on an exclusive basis the NIH’s intellectual property rights claimed for a Cooperative Research and Development Agreement (“CRADA”) subject invention co-developed with the Company, and the licensed field of use, which focuses on promoting anti-cancer activity alone, or in combination with standard anti-cancer drugs. The scope of this clinical research extends to checkpoint inhibitors, immunotherapy, and radiation for the treatment of cancer. The License Agreement is effective, and shall extend, on a licensed product, licensed process, and country basis, until the expiration of the last-to-expire valid claim of the jointly owned licensed patent rights in each such country in the licensed territory, estimated at twenty years, unless sooner terminated.
The License Agreement contemplates that the Company will seek to work with pharmaceutical companies and clinical trial sites (including comprehensive cancer centers) to initiate clinical trials within timeframes that will meet certain benchmarks. Data from the clinical trials will be the subject of various regulatory filings for marketing approval in applicable countries in the licensed territories. Subject to the receipt of marketing approval, the Company would be expected to commercialize the licensed products in markets where regulatory approval has been obtained.
The
Company is obligated to pay the NIH a non-creditable, non-refundable license issue royalty of $
The Company is obligated to pay the NIH, on a country-by-country basis, earned royalties of 2% on net sales of each royalty-bearing product and process, subject to reduction by 50% under certain circumstances relating to royalties paid by the Company to third parties, but not less than 1%. The Company’s obligation to pay earned royalties under the License Agreement commences on the date of the first commercial sale of a royalty-bearing product or process and expires on the date on which the last valid claim of the licensed product or licensed process expires in such country.
The
Company is obligated to pay the NIH benchmark royalties, on a one-time basis, within sixty days from the first achievement of each such
benchmark. The License Agreement defines four such benchmarks, which the Company is required to pursue based on “commercially reasonable
efforts” as defined in the License Agreement, with deadlines of October 1, 2024, 2027, 2029 and 2031, respectively, each with a
different specified benchmark payment amount payable within thirty days of achieving such benchmark. The October 1, 2024 benchmark of
$
The Company is obligated to provide annual reports to the NIH on its progress toward the development and commercialization of products under the licensed patents. These reports, due within sixty days following the end of each calendar year, must include updates on research and development activities, regulatory submissions, manufacturing efforts, sublicensing, and sales initiatives. If any deviations from the established commercial development plan or agreed-upon benchmarks occur, the Company is obligated to provide explanation and may amend the commercial development plan and the benchmarks, which, subject to certain conditions, the NIH shall not unreasonably withhold, condition, or delay approval of any request of the Company to amend the commercial development plan and/or the benchmarks and to extend the time periods of the benchmarks.
| F-35 |
The
Company is obligated to pay the NIH sublicensing royalties of
During
the year ended December 31, 2024, the Company incurred costs of $
Moffitt. Effective August 20, 2018, the Company entered into an Exclusive License Agreement with Moffitt. Pursuant to the License Agreement, Moffitt granted the Company an exclusive license under certain patents owned by Moffitt (the “Licensed Patents”) relating to the treatment of MDS and a non-exclusive license under inventions, concepts, processes, information, data, know-how, research results, clinical data, and the like (other than the Licensed Patents) necessary or useful for the practice of any claim under the Licensed Patents or the use, development, manufacture or sale of any product for the treatment of MDS which would otherwise infringe a valid claim under the Licensed Patents.
On October 4, 2023, the Company received a counter-signed termination letter dated September 29, 2023 with respect to the Exclusive License Agreement dated August 20, 2018 between the Company and Moffitt, effective September 30, 2023. The Company and Moffitt agreed that no termination fee was due or payable by the Company, and Moffitt acknowledged that no payments are owed by the Company under the Agreement.
During the year ended December 31, 2023, the Company recorded a credit to operations of $ representing the reversal of obligations previously recorded with respect to the Exclusive License Agreement.
Other Significant Agreements and Contracts
NDA
Consulting Corp. On December 24, 2013, the Company entered into a consulting agreement with NDA Consulting Corp. for consultation
and advice in the field of oncology research and drug development. As part of the consulting agreement, NDA also agreed to have its president,
Dr. Daniel D. Von Hoff, M.D., serve on the Company’s Scientific Advisory Committee during the term of such consulting agreement.
The term of the consulting agreement was for one year and provided for a quarterly cash fee of $
BioPharmaWorks. Effective September 14, 2015, the Company entered into a Collaboration Agreement with BioPharmaWorks, pursuant to which the Company engaged BioPharmaWorks to perform certain services for the Company. Those services included, among other things, assisting the Company to commercialize its products and strengthen its patent portfolio; identifying large pharmaceutical companies with a potential interest in the Company’s product pipeline; assisting in preparing technical presentations concerning the Company’s products; consultation in drug discovery and development; and identifying providers and overseeing tasks relating to clinical development of new compounds.
BioPharmaWorks
was founded in 2015 by former Pfizer scientists with extensive multi-disciplinary research and development and drug development experience.
The Collaboration Agreement was for an initial term of two years and automatically renews for subsequent annual periods unless terminated
by a party not less than 60 days prior to the expiration of the applicable period. In connection with the Collaboration Agreement, the
Company agreed to pay BioPharmaWorks a monthly fee of $
| F-36 |
The
Company recorded charges to operations pursuant to this Collaboration Agreement of $
Netherlands Cancer Institute. On October 8, 2021, the Company entered into a Development Collaboration Agreement with the Netherlands Cancer Institute, Amsterdam (“NKI”) (see Note 5), one of the world’s leading comprehensive cancer centers, and Oncode Institute, Utrecht, a major independent cancer research center, for a term of three years. The Development Collaboration Agreement was subsequently modified by Amendment No. 1 thereto.
The
Development Collaboration Agreement is a preclinical study intended to identify the most promising drugs to be combined with LB-100,
and potentially LB-100 analogues, to be used to treat a range of cancers, as well as to identify the specific molecular mechanisms underlying
the identified combinations. The Company agreed to fund the preclinical study, at an approximate cost of
On
October 3, 2023, the Company entered into Amendment No. 2 to the Development Collaboration Agreement with NKI, which provides for additional
research activities, extends the termination date of the Development Collaboration Agreement by two years to October 8, 2026, and added
On
October 4, 2024, the Company entered into Amendment No. 3 to the Development Collaboration Agreement with NKI, which suspended Amendment
No. 2 and provided for a new study term of one year and starts upon the dosing of the first patient in the trial at a project cost of
During
the years ended December 31, 2024 and 2023, the Company incurred charges in the amount of $
MRI
Global. As amended, the Company has contracted with MRI Global for stability analysis, storage and distribution of LB-100 for clinical
trials in the United States. During the years ended December 31, 2024 and 2023, the Company incurred costs of $
The
Company’s aggregate commitment pursuant to this contract, less amounts previously paid to date, totaled approximately $
Specific Risks Associated with the Company’s Business Activities
Serious Adverse Events
The Company’s lead drug candidate, LB-100, is currently undergoing various clinical trials, and there is a risk that one or more of these trials could be placed on hold by regulatory authorities due to serious adverse events (SAEs) related to the Company’s drug candidate or to another company’s drug used in combination in one of the Company’s clinical trials. It is possible that the SAEs could be attributable to the Company’s drug candidate and could include, but not be limited to, unexpected severe side effects, treatment-related deaths, or long-term health complications. A dose given could result in non-tolerable adverse events defined as dose-limiting toxicity (DLT). When two DLTs occur at the same dose-level, that dose-level is considered too high and unsafe. Further treatment is only allowed at lower dose-levels that have previously been found safe.
| F-37 |
If an SAE or a pattern of SAEs is observed during the course of a clinical trial involving the Company’s drug candidate, the U.S. Food and Drug Administration (FDA), European Medicines Agency (EMA), or other regulatory authorities may issue a clinical hold, requiring the Company to pause or discontinue further enrollment and dosing in its clinical trial. It is also possible that the clinical trial could be terminated. Any of these actions could delay or halt the development of the Company’s drug candidate, increase development costs, and negatively impact the Company’s ability to ultimately achieve regulatory approval. Additionally, if an SAE is confirmed to be drug-related, the Company may be required to conduct additional studies, modify the study design, or abandon further development of the drug candidate altogether, which could materially impact the Company’s business, financial condition, and prospects.
The occurrence of an SAE and any resulting clinical hold could also harm the Company’s reputation with patients, physicians, health institutions, and investors, diminish its ability to attract clinical trial participants, and damage its ability to interest investors and obtain financing in the future. There can be no assurance that the Company will not experience such SAEs in the future or that any related clinical hold will be lifted in a timely manner, or at all.
The principal investigator of the colorectal study testing LB-100 in combination with atezolizumab (Roche PD-L1 inhibitor) is currently investigating two SAEs observed in the clinical trial that was launched in August 2024. The Netherlands Cancer Institute (“NKI”) Institutional Review Board (the “IRB”) has put the colorectal cancer study on hold. The adverse reactions that developed in the two patients were dyspnea (shortness of breath) due to lung toxicity possibly or probably related to the combination of LB-100 and atezolizumab in one patient and fever and aphasia possibly or probably related to the combination of LB-100 and atezolizumab in the second patient. The patient who developed lung toxicity deceased due to the combination of lung metastases of colorectal cancer and dyspnea. The patient with fever and aphasia fully recovered from the adverse events with supportive medication.
Given the identified adverse events in the two patients in the clinical trial, the IRB requested from the principal investigator of the study at the NKI information as to whether the adverse events could have been caused by the combination of LB-100 and atezolizumab and information about the mode of action of the combination of LB-100 and atezolizumab. The principal investigator is preparing a response to the IRB detailing the safety experience with LB-100 given alone and in combination with other cancer drugs, especially doxorubicin and dostarlimab. Doxorubicin is a well-known chemotherapy, and dostarlimab is a well-known immunotherapy of which the mode of action is closely related to that of atezolizumab.
The reported adverse events in the colorectal cancer study have not been seen in any other patients thus far treated with LB-100 alone or in combination with other cancer drugs. Through February 2025, a total of 78 patient have received or are receiving experimental treatment with LB-100. It is expected that it will take at least two months to prepare a detailed response to the IRB, during which time the Company intends to update the safety overview of LB-100.
Other Business Risks
Covid-19 Virus. The global outbreak of the novel coronavirus (Covid-19) in early 2020 led to disruptions in general economic activities throughout the world as businesses and governments implemented broad actions to mitigate this public health crisis. Although the Covid-19 outbreak has subsided, the extent to which the coronavirus or any other pandemics may reappear and impact the Company’s clinical trial programs and capital raising efforts in the future is uncertain and cannot be predicted.
Inflation and Interest Rate Risk. The Company does not believe that inflation or increasing interest rates have had a material effect on its operations to date, other than their impact on the general economy. However, there is a risk that the Company’s operating costs could become subject to inflationary and interest rate pressures in the future, which would have the effect of increasing the Company’s operating costs (including, specifically, clinical trial costs), and which would put additional stress on the Company’s working capital resources.
Supply Chain Issues. The Company does not currently expect that supply chain issues will have a significant impact on its business activities, including its ongoing clinical trials.
| F-38 |
Potential Recession. There are some indications that the United States economy may be at risk of entering a recessionary period. Although unclear at this time, an economic recession would likely impact the general business environment and the capital markets, which could, in turn, affect the Company.
Geopolitical Risk. The geopolitical landscape poses inherent risks that could significantly impact the operations and financial performance of the Company. In the event of a military conflict, supply chain disruptions, geopolitical uncertainties, and economic repercussions may adversely affect the Company’s ability to conduct research, develop, test and manufacture products, and distribute them globally. This could lead to delays in product development, interruptions in the supply of critical materials, and delays in clinical trials, thereby impeding the Company’s clinical development and commercialization plans. Furthermore, the impact of a conflict on global financial markets may result in increased volatility and uncertainty in the capital markets, thereby affecting the valuation of the Company’s publicly-traded shares. Investor confidence, market sentiment, and access to capital could all be negatively influenced. Such geopolitical risks are outside the control of the Company, and the actual effects on the Company’s business, financial condition and results of operations may differ from current estimates.
Cybersecurity Risks. The Company has established policies and processes for assessing, identifying and managing material risk from cybersecurity threats, and has integrated these processes into its overall risk management systems and processes. The Company routinely assesses material risks from cybersecurity threats, including any potential unauthorized occurrence on or conducted through its information and email systems that may result in adverse effects on the confidentiality, integrity, or availability of the Company’s information and email systems or any information residing therein. The Company conducts periodic risk assessments to identify cybersecurity threats, as well as assessments in the event of a material change in the Company’s business practices that may affect information systems that are vulnerable to such cybersecurity threats. These risk assessments include identification of reasonably foreseeable internal and external risks, the likelihood and potential damage that could result from such risks, and the sufficiency of existing policies, procedures, systems and safeguards in place to manage such risks. The Company has not encountered any cybersecurity challenges to date that have materially impaired its operations or financial condition.
The Company is continuing to monitor these matters and will adjust its current business and financing plans as more information becomes available.
9. Subsequent Events
The Company performed an evaluation of subsequent events through the date of filing of these consolidated financial statements with the SEC. Other than as described below, there were no material subsequent events which affected, or could affect, the amounts or disclosures in the consolidated financial statements.
Nasdaq Compliance
On
August 23, 2024, the Company received a letter from the Listing Qualifications Department (the “Staff”) of the Nasdaq Stock
Market LLC (“Nasdaq”) on August 19, 2024 indicating that the Company was not in compliance with the minimum net stockholders’
equity requirement of $
On October 3, 2024, the Company submitted a plan to the Staff to regain compliance with the Stockholders’ Equity Requirement, which outlined the Company’s proposed initiatives to regain compliance by raising equity capital through various registered equity offerings.
On October 21, 2024, the Staff provided notice (the “Notice”) to the Company that it had granted an extension through February 18, 2025 to regain compliance with the Stockholders’ Equity Requirement, which required that the Company complete its capital raising initiatives and evidence compliance with the Stockholders’ Equity Requirement through filing a Current Report on Form 8-K with the Securities and Exchange Commission (the “SEC”) providing certain required information.
As of February 18, 2025, the Company had not gained compliance with the Stockholders’ Equity Requirement. Accordingly, on February 19, 2025, the Company received a Staff determination letter from the Staff stating that the Company did not meet the terms of the extension because it did not complete its proposed financing initiatives to regain compliance.
| F-39 |
The Company timely filed an appeal and requested a Hearing before a Nasdaq Hearings Panel (the “Panel”), which has been granted. The Hearing request automatically stayed Nasdaq’s delisting of the Company’s common shares and warrants pending the Panel’s decision. Pursuant to the Nasdaq Listing Rules, the Panel has the discretion to grant the Company an additional extension through no later than August 18, 2025. At the upcoming hearing, the Company will present its plan for regaining and sustaining compliance with the Stockholders’ Equity Requirement for continued listing. However, there can be no assurances that the Hearings Panel will grant the Company an extension of time to regain compliance, or that the Company will be able to regain compliance during any extension period.
The Company intends to take reasonable measures available to regain compliance under Nasdaq’s listing rules and to remain listed on Nasdaq. However, there can be no assurances that the Company will ultimately regain compliance with the Stockholders’ Equity Rule, or be able to maintain compliance with all other applicable requirements for continued listing on Nasdaq. If the Company does not regain compliance with Nasdaq’s continued listing requirements within the time period permitted by Nasdaq, then the Company’s securities will be delisted from Nasdaq.
Termination of At-the-Market Sales Agreement
WallachBeth
Capital, LLC. Effective January 6, 2025, the Company entered into an At-the-Market Sales Agreement (the “Sales Agreement”)
with WallachBeth Capital, LLC (the “Agent”) pursuant to which the Company may offer and sell from time to time through the
Agent, acting as agent, shares of its common stock, $ par value per share, having an aggregate offering price of up to $
The
offering of shares of the Company’s common stock pursuant to the Sales Agreement was scheduled to terminate upon the earliest of
(i) the sale of the maximum dollar amount of shares of common stock subject to the Sales Agreement, (ii) the termination of the Sales
Agreement by the Company or the Agent, and (iii) the expiration of the shelf registration statement on Form S-3 (File No. 333-278874)
on the third anniversary of the initial effective date of such registration statement.
Sale of Securities Pursuant to Securities Purchase Agreement
On
February 11, 2025, the Company entered into a Securities Purchase Agreement (the “Purchase Agreement”) with certain purchasers
named therein (the “Purchasers”), pursuant to which the Company agreed to issue and sell, (a) in a registered direct offering
(the “Registered Offering”), an aggregate of shares (the “Shares”) of the Company’s common stock,
par value $ per share (the “Common Stock”), at an offering price of $ per share, and (b) in a concurrent private
placement (the “Private Offering”), warrants (the “Common Stock Warrants”) to purchase an aggregate of
The Common Stock Warrants and the shares of Common Stock underlying the Common Stock Warrants have not been registered under the Securities Act of 1933, as amended (the “Securities Act”), and have been issued in reliance on an exemption from the registration requirements of the Securities Act afforded by Section 4(a)(2) thereof. The Common Stock Warrants and the shares of the Company’s Common Stock underlying the Common Stock Warrants may not be offered or sold in the United States in the absence of an effective registration statement or exemption from applicable registration requirements. The Company has agreed to file a registration statement to cover the resale of any share of Common Stock issuable upon the exercise of the Common Stock Warrants by April 4, 2025. The Registered Offering and Private Offering are referred to herein as the “Offering”.
| F-40 |
The
Offering resulted in gross proceeds of $
If a Fundamental Transaction (as defined in the Common Stock Warrants) occurs, then the successor entity will succeed to, and be substituted for the Company, and may exercise every right and power that the Company may exercise and will assume all of the Company’s obligations under the Common Stock Warrants with the same effect as if such successor entity had been named in the Common Warrant itself. If holders of shares of the Company’s Common Stock are given a choice as to the securities, cash or property to be received in such a Fundamental Transaction, then the holder of the Common Stock Warrants shall be given the same choice as to the consideration it would receive upon any exercise of the Common Stock Warrants following such a Fundamental Transaction. Additionally, as more fully described in the Common Stock Warrants, in the event of certain Fundamental Transactions, the holders of such Common Stock Warrants will be entitled to receive cash consideration in an amount equal to the Black-Scholes value of the Common Stock Warrants on the date of consummation of such Fundamental Transaction.
On
the Closing Date, the Company issued to the Placement Agent, or its designees, warrants (the “Placement Agent’s Warrants”)
to purchase up to
The Placement Agent’s Warrants and the shares of Common Stock underlying the Placement Agent’s Warrants have not been registered under the Securities Act and have been issued in reliance on an exemption from the registration requirements of the Securities Act afforded by Section 4(a)(2) thereof. The Placement Agent’s Warrants and the shares of the Company’s Common Stock underlying the Placement Agent’s Warrants may not be offered or sold in the United States in the absence of an effective registration statement or exemption from applicable registration requirements. As soon as practicable (and in any event by April 4, 2025), the Company has agreed to file a registration statement on Form S-1 providing for the resale by the Purchasers of the Common Warrant Shares issued and issuable upon exercise of the Common Warrants. The Company is obligated to use commercially reasonable efforts to cause such registration statement to become effective within 120 days following the Closing Date and to keep such registration statement effective at all times until no Purchaser owns any Common Warrants or Common Warrant Shares issuable upon exercise thereof.
Other Significant Developments
Effective
March 11, 2025, the Company entered into Amendment No. 1 to the Collaboration Agreement between the Company and GEIS that relieved the
Company of the financial obligation to support the randomized Phase 2 portion of the clinical trial contemplated in the Collaboration
Agreement of approximately $
| F-41 |